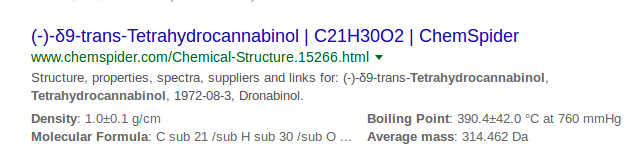
I use 1g/ml as an approximation for the density of crude all the time, and it’s pretty clear that I’m not alone on that one.
Crude floats on water, so it’s also clear that this is an approximation.
I’ve got 0.96 to 0.98g/ml in my head, and wondered if anyone had more solid data.
or I could just ask the all knowing one… which suggests 1.0g/ml isn’t bad at all.
6 Likes
Yeah, thats pretty close. Personally I use the 1ml/gr numbers for easy mathing! The lipid content will effect density as well.
2 Likes
Beaker
January 6, 2019, 7:23pm
3
I wonder how they derived the boiling point given the wide range of error they cite?
2 Likes
I’d be interested to know the bp at Atmos and room temp
you mean at 760mm Hg?
420C (390.2 +/- 42)
isn’t the boiling point at rm temp rm temp?
I’m an idiot I just saw the pic says 760mmhg lol
Is that accurate? 390c at atmospheric?
with +/- 42C “accurate” might be a stretch
420C, while obviously not accurate either, is certainly close, and easy to remember.
3 Likes
I’ve done some density measurements of ∆9 THC distillate before, ~85% ∆9. The density varied with temperature and was measured at ~1.032 g/cm^3 @ 20°C , ~1.008 g/cm^3 @ 60°C, and ~0.986 g/cm^3 @ 90°C. This was with distillate and not with crude oil but I imagine the values would be similar anyways.
1 Like