tbh, the meters don’t quite accomodate for this. That being said, it isn’t the biggest deal…
The meters count magnetic pulses as the gears rotate. The display unit then converts the number of pulses based on a “k factor” which is basically whatever multiplier is required for the given solvent.
butane is ~5p per gallon… propane… 5.75p per gallon. so as the blend shifts 10-20% there is a bit of inaccuracy built in, but the accuracy is not a large enough % to drastically fuck your data.
as a lab we struggled for a long time with maintaining our desired blend… did we need to add more butane as it had a lower boiling point and was more likely to get trapped in the columns/more likely to reside in the actual solvent being poured… Did we need to add more propane as when we off-gassed it was more likely to bleed out with the nitro?
seems like the only way to accurately gauge the blend in the solvent ratio is via a temp probe/pressure gauge. WIth those two data points, you can use one of these nifty charts:Propane Butane Mixture - Evaporation Pressure
an establish your ratio. And then adjust accordingly.
6 Likes
we always ordered solvent in groups of app. 45 tanks (before the hot vapor loop mod). In a 2 parts 70/30 to one part pure tane ratio. So we can top off the pure butane to help reestablish the ratio.
Had the most gangster relationship with a solvent supplier who would have his driver meet us in town. Swap cars, count the loot, and then let me take the company box truck…(after removing all hazmat placards and decals). and drive it ourselves to our worksite… unload/reload the truck and then return it like 2 hours later. Always tried to include a nice tip for the driver. Not sure the company would want me name dropping though.
dude even let us know when they switched gears and implemented some gps/dashcam shit so we could form a different plan. The company is on here. so if you’re reading this… 


9 Likes
Man… thought this would inspire more visionaries.
I’m getting calls from hemp processors, falling film users, and even some co2 guys. Anyone have anymore out of thr box ideas?
2 Likes
Do you void your engineer cert when you modify equipment?
depends on your relationship with the engineer.
short answer: yes.
although in this particular case the OEM is supplying the parts for the mods, after their engineer has determined that I’m not actually out in left field.
3 Likes
You are adding a pump to recirculate solvent that has already been through the material to get a more complete extraction?
1 Like
Works with Ethanol & C6/C7. Betting it’s worth a try with with C4.
EtOH documented here: It washes the cannabis and then it washes the cannabis some more
Heptane here: Help setting up a higher output extraction station - #21 by SidViscous
As @thesk8nmidget noted, OEM is sending a flowmeter.
Along with the pump I mentioned.
So your mileage may vary on how far you can vary before you need a variance from an engineer…
5 Likes
You need a density meter in order to measure saturation of a given solvent. Look to Coriolis Flow Meters.
3 Likes
Good point.
I wanted flow. And have a meter coming.
I also wanted saturation, but it’s not clear from Fraction Finder Reviews if the tool I was hoping to use will work.
Viscosity also changes with temp, and I’d rather control on “depleted” than “saturated”.
2 Likes
To be pedantic about it (aren’t I always?) a Coriolis meter doesn’t necessarily tell you when your solvent is saturated with cannabinoids, just when you’re no longer picking up anything new from your biomass.
Unless you’re dissolving pure cannabinoids only, of course.
But its a much better proxy than many of the other options out there.
And when you start talking reasonably large flows and pressures, they can actually be quite economical relative to positive displacement type meters.
But any measurement is better than no measurement.
5 Likes
In my experience, a flow meter with no mechanical moving parts will last when dealing with oils even in solution.
Just like any kind of monitoring device, it’s all about how you use that data. With the right density profiles it can be dialed in to be beneficial.
2 Likes
Prior to jumping into the BHOmeter project wholeheartedly we tested quite a few meter options as well as different places throughout the system.
For a long time… because I had two coriolis options, a -20 mechanical, and the original version of our Meter which was a -40c at the time…
-I had an oval gear -20c after our crc(if you read the B(arn)HO post I mentioned warming up our solvent post column…pre crc…) to measure rhe flow rate leaving rhe powders in an attempt to cross reference the impact residence time had on batch quality. I never encountered any issue with the meter. The only way oil suspended in the solvent could impact a mechanical gear/positive displacement meter would be if the viscosity was such that the flow rate dropped below its operable range, and I suppose possibly by way of “locking” the gears if a shitload of oil residue was left behind on a run. In a scenario like that though… it would just have to be mandatory to flush a little clean solvent through at the end of a run. and/or the solvent from the next run could. most likely dissolve any resistance prior to the gears spinning/solvent moving.
I tried rotating the mechanical gear -40(the first edition BHOmeter was a -50c… but otherwise the same unit) and the two coriolis options between injection-pre columns and recovery-post coils and didn’t initially experience much of a distinction. Over time, ironically, the more expensive of the two coriolis options ended up dropping in accuracy, but there was no way to tell while we were running. At some point, just for kicks, we put all three meters in line on the injection and noticed that the BHOmeter and cheaper coriolis were in sync… the other coriolis was reading way off. So we inferred that the “way off” coriolis needed to be calibrated. Which wasn’t particularly difficult perse, but could be majorly detrimental to the bottom line if one didn’t regularly test/calibrate the coriolis meters…
We thought we’d take the best of both worlds… Some coriolis stuff can handle much lower temp ranges, but the mechanical, oval gear tech is more reliable… So we found a manufacturing partner who would redesign/recertify their oval gear tech to the -90C range. (price point was also a factor… $4-5k is much easier ot swallow than 12-15k for most labs.)
We’re currently testing/negotiating with a new(to us) coriolis manufacturer though… As some claim that certain coriolis models can effectively measure entrained gas (liquid solvent with some vapor mixed in). and we have a.couple equipment manufacturers that specifically asked us to help them go about measuring it.
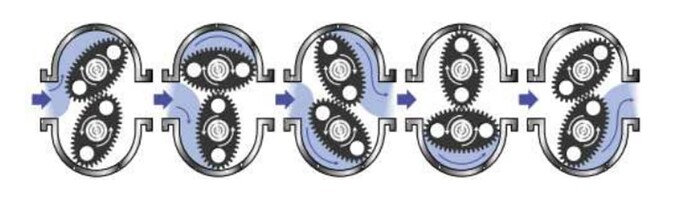
For those that don’t know… Here is a cool diagram of how an oval gear flow meter works. The jist of it is there are magnets embedded in the oval rotors and the machining is done very precisely… To the point that the interlocking gears create a seal. (Why you want to make sure a mechanical meter is of good quality). Poor machining/tolerances, and liquid can travel through the middle of the gears, resulting in opposing pressure so they don’t spin properly and therefore don’t track rotations and in turn, volume. Rotations are then converted via the correct “k factor” (individual to the meter and the solvent)… into a unit that your operators are familiar with.
5 Likes
Gear meters are definitely my preference for flow measurement at most reasonable flow rates.
I’ve just finished specifying one that looks a lot like yours for a related system. If you get good ones, which I think you have, they’re reliable and accurate and basically maintenance-free, which is a great combination.
Sure beats “shine a light on it and hope you learn something” meters.
2 Likes
Does anyone have or has anyone even determined the molar extinction coefficients for UV absorption at some given wave length…for the
“purified cannabinoic acids”? Somewhere along the line (including all the medusa stone bs) , someone has to say…this 99.5% plus chalk is what I
am going to define as my standard. But these COAs are a bit hard to believe in, due to the 4-cooh variant co-produced by plant…but some where it would be nice to “define an industry standard” for THCA purity…and everyone could work from there…so the term “pure cannabinoid” has some meaning. Moreover, there has to be a defacto definition of cannabinoic acid R-COOH form…a procedure where whatever you have in crystalline form…you subject to an acid wash step…and then recrystallize and deep vacuum dry.
Also at each node where one places a meter…the solution characteristics are changing due to increasing purity…as one approaches the above defined “pure cannabinoic acid level” …and of course different for all choices of solvent or mixtures of solvent.
@Bharris if you are serious about contending with the multivariate analysis in primary extraction data there is an academic paper published on cannabinoid extraction and variables that utilizes AI, a deep learning- perceptron approach to optimizing extraction conditions.
1 Like
The important question is not: what do you know?
It is rather: how do you know what you know? And what causes you trust that you do in fact know what you think you know?
This is a question that is unfortunately recursive and easy to get lost in.
7 Likes
Yes: “Butane works but no theory”
and out of that black box comes thousands of
comments. thousands…
well it seems no one even knows what “butane” really is or exactly where it comes from?
Yes and gear meters work well until you pump rescorcinol glue through it.
At least rescorcinol doesn’t stick to photons.
2 Likes