^ Ammonium Sulfate ^
Summary
Its possible since late 2020 an increase in MDEA (Tertiary Amine) use attributed to higher Ammonia content in treated LPG products; the Ammonia would then react with other contaminants resulting from the degradation of the DEA/MEA (Primary or Secondary Amine) which may have always been there as per usual norm, but with more MDEA recently may likely be forming the compound of interest due to increase of Ammonia from source sour crudes.
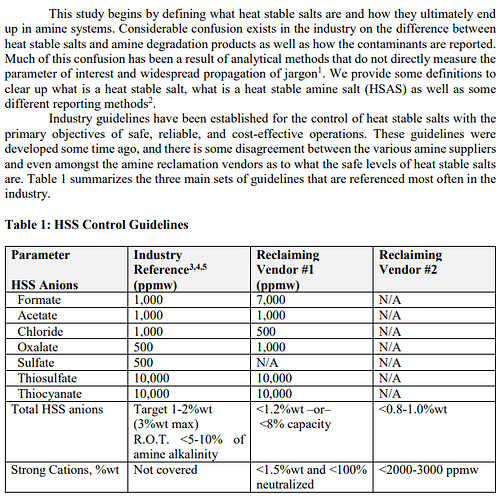
See bottom left four cells of Industry Ref. + Vendor #1 column
/
/
Difference between Primary/Secondary and Tertiary Amines
- When using MEA, removes the CO2
Thermal degradation of MEA becomes a problem when introduced to high temperatures which can occur in the Reboiler, and causes localized overheating. This decomposes the MEA and apparently produces Ethylene Oxide which can be polymerized and react with the MEA, kind of interesting, and or any anti-foam agents used. The products of the reactions can be solid, or liquid, deactivates the MEA in solution, and favors the effect of foaming.
The presence of the following amine degradation products increases the foaming coefficient (listed in order of increasing foam volume): Ammonium Thiosulfate, Glycolic Acid, Sodium Sulfite, Malonic Acid, Oxalic Acid, Sodium Thiocyanate, Sodium Chloride, Sodium Thiosulfate, Bicine, Hydrochloric Acid, Formic Acid, Acetic Acid, and Sulfuric Acid.
- When using MDEA, removes the H2S
Good benefits for use in Merox treating; by being nonreactive, it allows for CO2 slip (but sometimes there are issues, read on), so more amine is available for H2S pickup and solvent rates can be greatly reduced. With reduced solvent rates come lower Reboiler heat loads, reduced solvent pumping requirements, and smaller columns.
Before make up addition of more MDEA, its weak and thus some of the disulfides from the process carry though via incomplete oxidation, which form Thiosulfate.
/
/
Primary / Secondary + Tertiary Amines
MDEA inhibits the collection of Ammonia, while DEA/MEA degrades and outputs other contaminants that cause reaction formulations to occur, the specific of interest being Ammonium Thiosulfate? or Sulfuric Acid and Ammonia which would yield Ammonium Sulfate?
Monoethanolamine, and Diethanolamine, are oxidized from Methyl Diethanolamine, you need a Primary or Secondary amine to deal with the CO2, and the Tertiary (MDEA) to deal with H2S preferentially, at least if you’re trying to be profitable that is, or the input feed is one way or the other leaning.
/
/
Amine Carryover aka Issues in the Stripper
In the Stripper, the equilibrium value for Ammonia from source feed into the Condenser is considerably < 1, causing Ammonia vaporized in the Stripper to be returned into the Reflux. The equilibrium value for Ammonia within the Stripper is a bit > 1, resulting in a mild tendency to exit the Stripper overhead rather than the bottom. So the Ammonia concentrations tend to build up in the Stripper as more Ammonia tries to exit the top, which causes it to be collected and returned into the Reflux again.
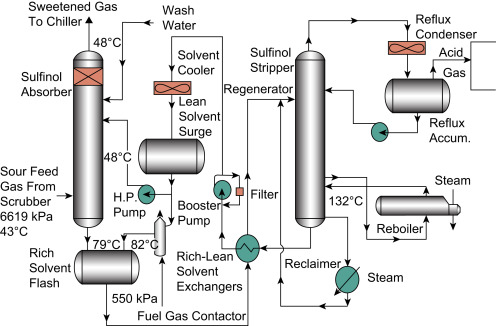
Concentrations increase until the amount of Ammonia surpasses the low equilibrium value within the Condenser, or forces its way into the Reboiler against high equilibrium value in the Stripper, which would then carry on into the make up water and cooling line before reintroduction back into the top of the Absorber, where it exits out into the treated products.
/
/
Conclude
It kinda seems like the CO2 slips up the Stripper but not before letting a lot of the NH3 go in the reboiler which I believe uses in contact steam and “washes” the “treated caustic” (and NH3) before going back into the top of the Absorber again, and seems like the refineries had to make some kind of operational compromise or pivot and likely this could be it, and its safe to assume the process parameters are no where near ideal at this time.
/
/
Testing Standards
ASTM D1835-18a.pdf (633.1 KB)
Have identified the most relevant testing standards by ASTM and ISO, now asking the suppliers to identify which standards they meet and if they can confirm what standards the refinery that supplies them. Of all the standards I looked at, none of them seem to indicate a way to quantify these byproduct reaction contaminants? The table posted on the top shows some “industry standard”, at which point you can see there is considerable wiggle room in the “Thiosulfates” so, perhaps, the trouble compound is evading detection by any quality control standards and again, only we would notice… something to do with THC acid specifically and formation of polymerized compounds or emulsion.
Copper Strip Test for Salts: ASTM D1838__12a.pdf (117.0 KB) MUST BE 1B RESULT
Documents Read for this Reply
Contaminants Generated by Aqeous Amine Solutions.pdf (4.8 MB)
Contamination in Amine Systems.pdf (682.9 KB)
Effect of Heat Stable Salts.pdf (410.2 KB)
Effect of Heat-Stable Salts on Amine Absorber and Regenerator Performence.pdf (208.6 KB)
Fate of NH3 in Refinery Amine Systems.pdf (928.1 KB)
Heat Stable Salt - Terminology.pdf (39.4 KB)
Influence of Ammonia on Gas Sweetening Units Using Amine Solutions.pdf (153.7 KB)
Mechanism of Formation of Heat Stable Salts.pdf (340.0 KB)
Drilling Muds - Guide to Oil Field Chemicals and Fluids.pdf (724.5 KB)
Dynamic Operation of Liquid Absorbent-Based Post-Combustion CO2 Capture Plants.pdf (722.5 KB)
BONUS
@hambread
Found this for you, I don’t know if it helps…you may know already;
Some corrosion inhibitors (eg, sodium metavanadate and copper carbonate) can reduce the solution surface tension and increase foaming tendency, where as sodium sulfite has no apparent affect. Surface tension typically increases in the presence of small halogen anions, large cations, and divalent anions. Conversely, acetate ions and large halogen anions act as surfactants and reduce surface tension. Thus, an important factor in foaming tendency is the potential introduction of ions or salts that change surface tension.
- Any species which tends to decrease the surface tension of the solution will increase the tendency to foaming.
- The presence of surface-active, finely divided solids or polymers can strongly foam the system.
- Any species which tends to increase the viscosity of the solution will stabilize formed foam.
Thanks for reading, let me know your thoughts. I really could use someone more informed than myself to confirm any of this theory if possible, of course we’re all busy.