Hello, I am attempting to up my winterization game by understanding what I am filtering out at what temps. Does anyone here have a link they would be willing to share with me on the melting/freezing points of the various constituents of crude extract?
Interesting, never thought about looking into it further…
What I have seen,
-
Roughly 50% of the components precipitate out of a 190 proof ethanol/crude solution at room temperature
-
The vast majority, often all, of the remaining winterizeable components precipitate out of solution after 12 hours at 0c
-
Occasionally there are some components that wont precipitate until the solution is brought down to much colder temper (Dry Ice sublimation at ~ -70c)
Thank you for your insight. I am also curious to know if the saturation level of crude/ethanol solution plays a role in the melting/freezing point of said components?
I have not seen any data to quantify this, but it would make sense that a diluted solution would prevent precipitation in its full form. I would suggest experimenting with the same material and try winterization at different levels of dilution (1:5, 1:10) etc. Let them sit at room temp for a few hours and then filter. Weigh the filter paper before and after to get an idea of how much you pulled.
Will try this myself and report back.
Thank you
Hello. I recommend this procedure which was shared to me by O.S.
This is a work in progress so I appreciate any comments. Solvent of choice, time, temperature, dissolution rate are some of the many factors that need to be studied. This will vary with extraction technology used and variations may occur even within strain.
How to determine percentage of wax in crude:
- Start with 100 grams of crude, dissolve in solvent as per your SOP.
- Record the weight of your solution (ie 1,100 grams)
- Cover your equipment to prevent evaporation.
- Freeze for time/temperature of standard SOP.
- Weigh filter paper to be used.
- Weigh and label all glassware to be used.
- Filter as per standard SOP.
- Weigh filtered solution.
- Weigh wax and paper.
- Let wax/filter paper dry for 24 hours in a well-ventilated area.
2 data sets are generated:
The solvent filtered minus the original tincture weight is your approximate wax %. For example, beginning weight was 1,100 grams and ending weight was 1,085 grams. 25 grams were removed from solution, which is wax & other lipids + solvent.
Filter paper, for illustration purposes weighs 1 gram. The wax filtered (including paper) should weigh 26 grams (wax filtered + paper). If it weighs less, loss is calculated ass solvent loss. For example, the filter paper, solvent and wax weigh 25 grams, not 26. In this case 1 gram of solvent was lost or 0.1% of weight. Once the wax dries, the total weight reduces to 16 grams (again, just hypothetically). 9 grams were lost to evaporation, thus total solvent loss was 10 grams or 1% by weight. The remaining weight, 16 grams is wax + paper thus you only have 15 grams of wax. 15 grams of wax out of 100 grams of crude is 15% wax.
This data is important as it is essential in calculation the size of equipment needed to provide an effective filtration solution.
How to determine dissolution rate:
The solvent used to dewax, as well as the rate the crude is dissolved at are critical for high quality crude. Generally, filtration is more efficient at higher dissolution rates.
Run the following tests with several solvents (Iso, Ethanol, Methanol, etc…) to determine what solvent to use and at which dissolution rates. Every cannabis strain is different, and we recommend these tests as part of your quality control procedure in order to sustain quality extracts.
- Start with 20 grams of crude, dissolve in solvent of choice (perhaps ethanol) at a ratio of 5 parts solvent to 1 part crude.
- Repeat with 20 grams of crude, dissolve in same solvent at a ratio of 10 parts solvent to 1 part crude.
- Repeat with ratios of 20:1 and 40:1.
- Record the weight of your solution (ie 220 grams)
- Cover your equipment to prevent evaporation.
- Freeze solution at -67C for 24 hours.
- Weigh filter paper to be used.
- Weigh and label all glassware to be used.
- Filter using 1 micron filter paper or similar.
- Weigh filtered solution.
- Weigh wax and paper.
- Let wax/filter paper dry for 24 hours in a well-ventilated area.
- Determine which dissolution rate generated the most wax. This is the preliminary SOP for this particular crude.
- Once a good ratio is selected you may begin to dial down to perhaps conclude a ratio of 12:1 is the best for a particular crude by trying intermediate dissolution rates.
Repeat all tests with each solvent and compare results.
You may wish to try freezing on a normal -20C freezer to compare results.
You may also wish to study the effects of filtering your solution immediately after removing from refrigeration, as well as letting the solution warm up for 30 mins (or varying times) at room temperature.
When the fats supersaturate the solvent, they precipitate (crash) out of the solution.
Temperature and solubility are connected. Warm ethanol will dissolve more “fats” than cold ethanol can carry.
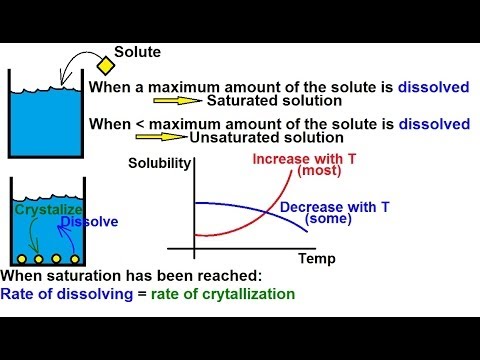
You can see that effect in the red line on the graph.
With that in mind, I’ve found that, with the amount of fats that drop out of ethanol (190 proof as per @Photon_noir recommendation) at room temp, it can dramatically speed up your overall filtration process to filter those fats out prior to crashing the entire solution to sub zero temperature.
@SamboCreeck.com this would be the only addition to your very thorough methodology
How long do you usually leave it at room temp to crash off the first bit of fats before filtering the first time and subzero freezing?
Material dependent… 10 minutes to 6 hours
Love this reply. Thanks for the enlightenment.
yes but fractional precipitation does exist. ive just never good data specific to cannabis constituents
Indeed
How does this SOP change when extracting with Dry Ice?
You mean extracting cannabinoids from flower with dry ice chilled ethanol? Or using dry ice to freeze fats out of a fatty ethanol solution?
I prechill the ethanol before extraction. I’ve always wondered if I really need to go through any of these steps.
At -40c I’ve found that a 10minute soak with 190 proof ethanol extracts the majority of the cannabinoids, and few enough lipids to not require winterization
This is how I’ve always done it room temp first then freeze.
Yeppir! Using 190 proof (the exact ethanol:water azeotrope) is perfect for keeping cannabinoids dissolved, even at extremely low temperatures, while precipitating all the epicuticular waxes at room temperature, then all the vegetable oil at reduced temperatures. The azeotrope is special, because as soon as you change it, you can see the solubilities rapidly changing. By adding pure water, for example, the cannabinoid resin will “louche” (i.e. precipitate as a liquid/liquid microemulsion similar to milk). If you have the “water waxes” already precipitated and floating in your solution at room temperature and you add pure ethanol, you will see the waxes disappear into solution. That perfect water balance makes a big difference in the quality of your Winterization! ![]()
does this mean that i can skip winterizing altogether? straight to rotovap the n decarb?
Yes. -67c is the sweet spot according to @Photon_noir but I have good success at -40c