@TwistedStill - its a great idea; I’m wondering (in my tiny peanut brain) if there’s off the shelf equipment from beer/wine mfg (or?) that would accommodate more head space for a larger batch ?
Your always welcome to meme my friend.
Any reaction that has a phase change from solid or liquid to gaseous will be affected by pressure.
Because decarbing involves the liberation of CO2 in the gaseous phase, increasing pressure will slow the reaction.
At a high enough pressure, it would probably be possible to carboxylate THC.
This gets complicated because of the critical properties of CO2 but is correct in concept.
As I remember this is Le’Chatilers principle.
I think decarbing under moderate pressure to surpress terpene evolution is a good deal.
The reaction would probably go the other way at 1000 atmospheres.
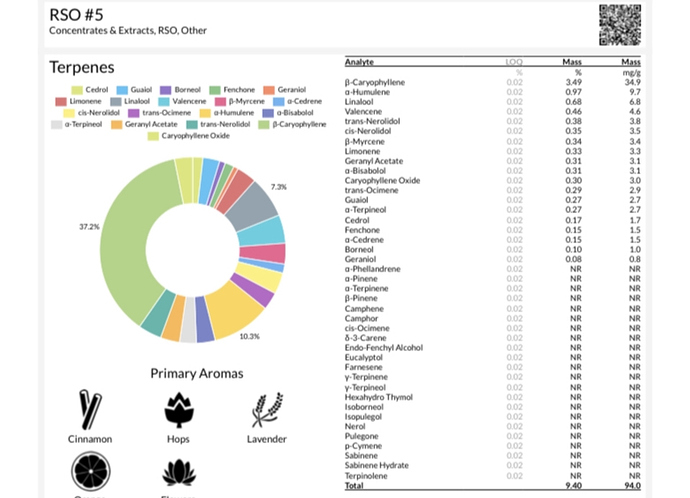
I didn’t notice a difference between 7psi of nitrogen and 45psi. I’m thinking pressure is less relevant that simply decarbing in an inert atmosphere in a sealed vessel.
I thought adding pressure could make the release of co2 slow. Thank you for that info. You all are why I am able to do this stuff. Your knowledge and experience has leveled me up beyond what I could have done alone.
I think we are talking hundreds of atmospheres before we would see an effect on the decarb reaction.
that has been argued against.
but at 1k atm I’m willing to consider it…
how about you @Photon_noir ?
can someone check my math on how important headspace & PRV’s are?!?
I showed my wife this meme…she is due any day now. She is going to use the meme for the announcement thing when the little dude is born. #memelife @Dred_pirate your meme lives on…
On a side note from the meme, the extra pressure seemed to do nothing to help in terpenes preservation. 7psi if I remember correctly on the first use, and 45psi on the second. I have no data to show this…this is just from knowing the strains I grow, and having experience with them.
But…the carts I made with the oil smell too much to have on my person. I carry one at all times…and 10 hours a day I cannot “smell like the dank” as a coworker so kindly put it. He was right…you can smell it quite a distance away. They are NSFW, at home use only. So that is a sign I’m moving in the right direction I suppose lol
Gaaah seeing that video makes me so glad i no longer work in co2 extraction… faaak
sorry if youve mentioned, what carts are you using? and how viscous is your oil?
Thanks for sharing
Ascent full ceramics on that round. The oil will move at room temp, but it will not move noticeably to the eye…unless you got time on your hands. Sorry, I have a hard time using words to describe the viscosity of thick oils.
Also on a side note…i made it to the grey wheel today to polish up some tools I squished.
I am honored that she’s going to keep it living on
Yeah we run a system at 1000 bar. It does not re-carb material on its own. Nor does it seem to decarb at accelerated rates, at least within 36 hours which is the longest I’ve maintained those pressures.
Well, yeah. All kinds of shit gets weird at super high pressures! Supercritical THC, anyone? Seriously. The cannabinoids are more likely to break down into coal at pressures over 10kpsi than to do anything useful… unless that’s what folks mean by “Straight FUEL, Fam!” ![]()
![]()
![]()
![]()
There are a bunch of great applications for these pressures, none of which I’m allowed to talk about lol. One of them rhymes with “crep phromatography”. In all seriousness, there’s a lot of talk about high pressures causing issues with reactivity and that simply isn’t what we’ve seen at all. The pressure certainly doesn’t seem to have anywhere near the affects of increased temperature.
You’re spot on, there, when it comes to 1st Order reactions and “pseudo” 1st Order reactions like decarboxylation (which I think is the rxn of topical interest here). However, pressure does change rxn speeds and equilibria with 2nd Order rxns like catalytic isomerization, which usually also involve some elevated temperature… although you are still correct that temperature plays a more significant role than pressure.
I’ve kept over 9% terps on ethanol crude decarbing in a mason jar. Badass work on your contraption though
nice work!
What temp, how long and how full was your jar?
Any reaction where the product is a gas will have some rate sensitivity to pressure changes.