[edit: note that MW for THCA is wrong here. Used what the all-gnowing handed me without thinking…]
maths?!?
I’ve tried to get someone else to do this in public multiple times.
guess I’ll give it a go…
lets call 1 mole of THCA 315g, and use the approximation of 1g/ml to get the volume of our concentrate when we started. Call it 315ml.
if we start in a 1liter container, and don’t deal with the fact that the density of our extract will change as we heat it, we can pretend we have a headspace of 685ml. lets evacuate that before we start, and pretend that we actually managed to empty it. [yeah, I’m cheating…]
how about we decarb at 100C?
mainly because it’s trivial to reliably achieve and maintain.
there’s the “how many liters of C02 a mole of THCA will produce”, which is 22.4liters at STP. which is 273K or some such.
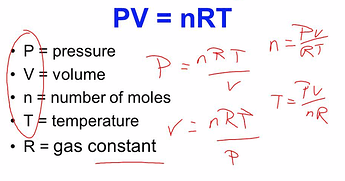
then we use PV=nRT to figure out how those 22.4l of CO2 are gonna fit in that 685mls at 100C [yep, cheating. some fraction of the terpenes will be in the headspace too!]
we have:
V=0.685L
n=1mol
T=373.14k
R=0.08206 atm L/mol.K
(lets ask the all knowing one to rearrange)
so we can get P = nRT/V
=> 0.08206*373.14/0.685 = 44.7 atm!
which the all knowing one says is 670PSI
sounds kinda** scary…
with only 31.5grams (just over and oz) in there, the math looks 10x better (ish)
0.1 x 0.08206 x 373.14 /0.9685 = 3.2 atm => 47PSI
which is why you want copious headspace.
or something that only holds some of the pressure.
** depends on your 1liter container.
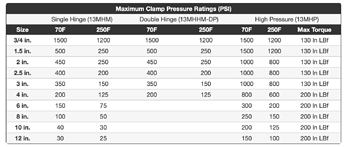
if using triclamps, here’s a reminder on pressure ratings.
Edit: @Photon_noir did the math differently (and some time ago). As posted in So I made a thing to decarb with.... - #58 by TwistedStill . results look pretty similar to me. Would love confirmation. Anyone?!?