To clarify, you do use the recovered ethanol again and again with no issue?
Yes, until it needs reproofed just like usual.
What do you mean by re-proofed and how is that performed? Is there an article you can share with me that details the process or where I can find it?
its bringing your solvent back to its original proof that it was when new, your most likely gonna recover water mo matter what. Theres a few ways to do it, google jt and see which way will work best for you
http://future4200.com/search?q=reproof
Or tell the boss you need to go to scotland for 6months to study ethanol distillation ![]()
After winterizing, filter fats and lipids, I use a hoxtrem filter with filter paper and glass wool. Pour filtered solution back into media bottles and do another filtration with charcoal. Then throw that solution into the roto and get as much but not all ethanol out so it’s easier to pour out crude into beaker. I pour 400ml into a beaker and place on a hot plate with stir bar and set temp at 280 which is max on my plate. You’ll see the excess ethanol react and fill to the top as it boils, simply stir it up and reaction will drop down. When that reaction stops and your crude is settled with minimal to no bubbling, it is fully decarbed and ready to distill. I doo 400ml in a 1kml beaker at a time because that amount is much easier to control. ![]() Hope that helps.
Hope that helps.
Does decarbing the oil oxidize it? Sorry im still learning ![]()
Yeeeeeee ![]()
You’re not gonna oxidize your cannabinoids as easily as you will your terpenes
What you say is for sure true but is all relative. If we are talking about making distillate, Terpenes shouldn’t really matter.
unless you oxidize them and don’t get them all out.
==> burnt rubber
upthread…
if you’re going for distillate, then as @FRESHcoastextracts said, you’re planning on removing the (far more fragile) terpenes anyway so its not a huge issue.
however, if you try hard enough, you can definitely do a number on your oil during decarb.
Couple passes on spd takes care of that in my experience. Would you agree?
haven’t distilled THC in a while. doesn’t seem to be as big an issue with CBD
Last time I tried removing that stench (from someone else’s first pass thc), a second pass alone did not solve it.
I reuse ethanol all the time, but i only winterize first pass after removing all the terps
Makes sense.
Ok, noooob question, plz be kind:
To avoid losing terps during decarb, can you seal in canning jar, heat for correct time duration, remove, cool?
I know heating sealed anything = boom. However I’ve been told by the wyze interweb this works. Just never tried it. Anyone here try this?
yep
- So I made a thing to decarb with
- Decarb under pressure to retain terps?
- So I made a thing to decarb with - #66 by Midnightoil
you might want to wrap your head around how many liters of C02 a mole of THCA will produce… or just leave copious head space ![]()
Edit:
some may not interpret this as kind, but I’m going to point out that the link to Decarb under pressure to retain terps? was given up thread as well.
not to give you shit for not following it, but to demonstrate why reading much of this site more than once can be beneficial.
[edit: note that MW for THCA is wrong here. Used what the all-gnowing handed me without thinking…]
maths?!?
I’ve tried to get someone else to do this in public multiple times.
guess I’ll give it a go…
lets call 1 mole of THCA 315g, and use the approximation of 1g/ml to get the volume of our concentrate when we started. Call it 315ml.
if we start in a 1liter container, and don’t deal with the fact that the density of our extract will change as we heat it, we can pretend we have a headspace of 685ml. lets evacuate that before we start, and pretend that we actually managed to empty it. [yeah, I’m cheating…]
how about we decarb at 100C?
mainly because it’s trivial to reliably achieve and maintain.
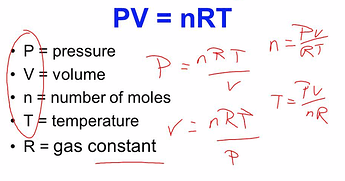
there’s the “how many liters of C02 a mole of THCA will produce”, which is 22.4liters at STP. which is 273K or some such.
then we use PV=nRT to figure out how those 22.4l of CO2 are gonna fit in that 685mls at 100C [yep, cheating. some fraction of the terpenes will be in the headspace too!]
we have:
V=0.685L
n=1mol
T=373.14k
R=0.08206 atm L/mol.K
(lets ask the all knowing one to rearrange)
so we can get P = nRT/V
=> 0.08206*373.14/0.685 = 44.7 atm!
which the all knowing one says is 670PSI
sounds kinda** scary…
with only 31.5grams (just over and oz) in there, the math looks 10x better (ish)
0.1 x 0.08206 x 373.14 /0.9685 = 3.2 atm => 47PSI
which is why you want copious headspace.
or something that only holds some of the pressure.
** depends on your 1liter container.
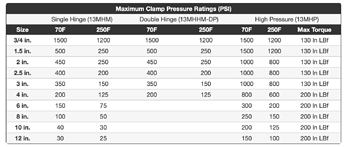
if using triclamps, here’s a reminder on pressure ratings.
Edit: @Photon_noir did the math differently (and some time ago). As posted in So I made a thing to decarb with.... - #58 by TwistedStill . results look pretty similar to me. Would love confirmation. Anyone?!?
Anyone else playing with decarboxylation to lower temp and speed along the process?