Possibly. That’s why I didn’t bother linking said information…
![]() the length they go to get you in
the length they go to get you in
There are actually several phenomena at work when distilling solvent from a solution @Griffin.Labs , especially with a high heat capacity, high viscosity and mixed solute like Cannabis resin!
You are correct that the solvent’s physical properties dictate the basic thermodynamics… Boiling point at a given pressure is a constant, and added heat does not change the boiling temperature; only speed of boiling, and therefore vapor pressure, as well as the rate of cooling, albeit perhaps counterintuitively.
However, because of the thick heavy solute, as solvent disappears and concentration of the high b.p. solute increases, the temperature can increase if you increase the heat input, because the rate of evaporative cooling by solvent boiling slows down! In other words, it would require faster heat input to achieve the same rate of boiling as the dilute solution, as the resin concentration increases… but that is unnecessary and probably deleterious to your terpene profile.
I recommend leaving the temperature/heat input the same, all the way to the end of your solvent recovery, because reducing the heat will just compound the slowing effects as resin concentration increases, and it doesn’t actually help to preserve more terpenes, anyway since, as noted, the temperature will remain fairly constant in the solution. In fact, reducing the temperature while actively pulling vacuum with a vapor compressor will just cause stronger vacuum while the solvent boils off slower (due to viscosity), possibly increasing the loss of terpenes by excessive time under even stronger vacuum! ![]()
I understand and agree with the boiling theory however at the end of the recovery once solvent is gone then we can heat the extract above a desirable temperature if the jacket temperature is high.
I think the way we are looking at this is a tiny bit too simplistic. There is a boundary layer of solution and as the solution reduces the boundary layer where solvent boils off tends to leave a residue or film on the heated walls of a jacketed vessel. This doesn’t happen to the same extent on old style systems like MKIII terps with just the bottom submerged in hot water. The phenomena only seems to occur on heated walls. I don’t see a ton of it on big iron fist collection vessels on the walls - just the heated coils. Unless the jacket is employed for heating and then some collects. Now for the remainder of recovery the oil adhered to the warm walls is sitting on heat and it’s much closer to jacket temperature than boiling point of solvent temperature.
You can rinse this residue off and lessen the loss to the walls by refluxing solvent towards the end of the run. However now you are rinsing oil (that has sat on heat without the cooling effects of the boiling solution) into the main body. The greater the heat in the jacket, the greater the theoretical impact to product quality.
Has anyone else thought much about this or am I again overthinking a basic concept? I have never tried making live resin on a jacketed collection with temps above 35C so I don’t know how higher heat does or doesn’t degrade product.
Does ur thca yield go down w straight propane run? Noticeably…its probably lighter…leave ton solvent for the pour and get it balls cold and u might can get passed the collection pot muffin…
I have extras tank w prvs I wanted to try this on… but other than better prvs.and even heavier duty clamp what makes system propane…thicker steel?
@Killa12345 I’ve asked this before but what we rated to
I wonder if u ran dry ice cold whole time no nitro could I swing it without dying
@StoneD propane is cool but you need a whole system rated to 350psi to safely handle pressure. I have heard of a fair amount of people deciding it would be ok to run propane in their rig and have suffered the consequences of not having their entire system rated to 350 psi from mild injury to near death experiences . The reason for this is you should never go above 175 psi in your Sop anyways but just in case you double it . Please do not do it unless you have expensive certified equipment or confirm with equtipment manufacturer . The risks are not worth the rewards it scares me personally . My friend told me a story he went to open the valve on a fresh 20# cylinder of propane to distill as soon as the knob opened it busted off and the whole tank dumped in 3 secs flat nothing he could do but evacuate slowly and open it up . This was just a lesson learned . Heard a story of a 12" clamp popping cause of propane and hitting someone across the face they are only rated to 100 psi . Guess the guy lived but is permanently scarred across the face
I think you meant about 1%. Unless you are using sludge.
So just to clarify, any system running pure propane would have to have 4" clamps or smaller? Unless you were to run the big ass ASME HP clamps? I’ve been thinking about upgrading to 6 inch but have been hesitant until I can get it all figured out for sure.
The only system I’ve used pure propane on is an ETS. I never had any issues with their hardware. The chilling methods they provide I won’t speak fondly of but their steel is solid. The clamps on that system we’re almost all 6 inch clamps. I would just suggest you look into what your clamps can handle before you ever even use them. And that goes for all clamps really
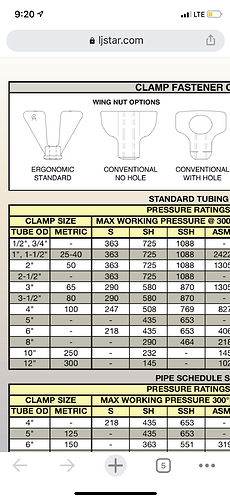
That is the part I’m confused about. I see systems like ETS, BizzyBee, N.B. Oler, all use 6" clamps. But all of the clamp specs I see only rate the 6" up to 300 PSI @70F while others on here are calling for 350 PSI. Even the clamps N.B. Oler offers which have a 1/2" bolt replacing the standard 3/8 say they are only rated to 300 PSI. LJ star is the only brand I can find that rates their 6" clamps above 350PSI. Does anyone know of other brands that have 6" clamps rated for propane?
LJ Star SSH are the only 6” clamps I use.
I have always heard of 6" being the standard for propane . Back in the day i was told to avoid cheap companies in particular if running a high propane blend or pure propane . Most cheap companies explicitly state for use with n-butane only . Doubling highest SOP is just a good rule of thumb to build a shit brickhouse . For example the the MVP 150 can be used with pure propane but only to 150 psi many people safely do it but it would be possible with an accident or operator error it could potentially go higher than 150 psi it would just be very unlikely . Another example is with a pressure vessel for holding liquid solvent if you fill over 75% on accident then apply heat if your not rated to 300+psi things could go south very quickly even with prv steel can warp or turn into a grenade so its better to build it more than less
The reason you only use LJStar SSH clamps is because you are a smart man, and that’s what I appreciates about you.
Here’s a cutsheet showing pressure ratings for their clamps.
If you didn’t want to put your tank in hot water, you could just use a little bump of nitrogen in the headspace of your tank.
So if anything happens to your ice it could destroy your system… I get what you’re saying, but I don’t think you should advise people to do something this dangerous
I think it’s quite exciting
Oh it would be. The end of that day would feel like 3 days without sleep
Propane is your best friend.
Where are you purchasing propane without mercaptans? I’ve purchased a 70/30 Butane/propane blend from BVV, but it’s not viable financially, utilities g new tanks and paying shipping, to spendy.
None of the gas suppliers in my area, will sell mercaptan free propane.
Can nitrogen be used to charge my Butane tank, bring the PSI up to 45 or 50 psi, up from the 12 psi that Butane typically has at 70F?
Yes, you can use nitrogen to increase the pressure in your butane tank. Provided your tank is rated for it. Don’t use compressed air though ![]()