Again, not claiming to be an expert in any capacity
3.4.1.2 Butane Isomerization
The purpose of butane isomerization is to generate feed material for a facility’s alkylation
or MTBE production unit; alkylation unit feed includes isobutane and olefins, while the raw
materials used in making MTBE are isobutylene and methanol. Butane isomerization is a much
older process than naphtha isomerization, having been used in refineries since World War II.
Presently, the most prevalent method of producing isobutane from n-butane is the UOP Butamer
process, similar in many ways to the isomerization of naphtha over platinum chloride catalyst.
In the Butamer process, normal butane, generated from throughout the refinery and separated
from other butanes by distillation, is combined with hydrogen and a chlorinated organic
compound. The hydrogen is used to suppress the polymerization of olefin intermediates, while
the chlorine source is used to maintain catalyst activity. The feed flows through one or two
fixed bed reactors in series, containing platinum chloride on alumina catalyst. The isobutane product is recovered and used as alkylation unit feed. Butane isomerization takes place at lower
temperatures than naphtha isomerization.
Also this is interesting
Although the Butamer process and others using platinum chloride on alumina as a
catalyst dominate the industry, other technologies are also used. Three facilities conducting
butane isomerization do not use platinum catalysts. Instead, the catalyst is aluminum
chloride/hydrochloric acid and generates an almost continuous spent catalyst waste stream in
slurry or sludge form
Then
3.6.1 Sulfuric Acid Alkylation Process Description
In the sulfuric acid alkylation process, olefin and isobutane gases are contacted over
concentrated sulfuric acid (H2SO4
) catalyst to synthesize alkylates for octane-boosting. The
reaction products are separated by distillation and scrubbed with caustic. Alkylate product has a
Research Octane Number in the range of 92 to 99. Figure 3.6.1 provides a generic process flow
diagram for H SO alkylation.
Alkylate product has a Research Octane Number in the range of 92 to 99. Figure 3.6.1 provides a generic process flow diagram for H SO alkylation. ← Purity higher than 92 must use this…
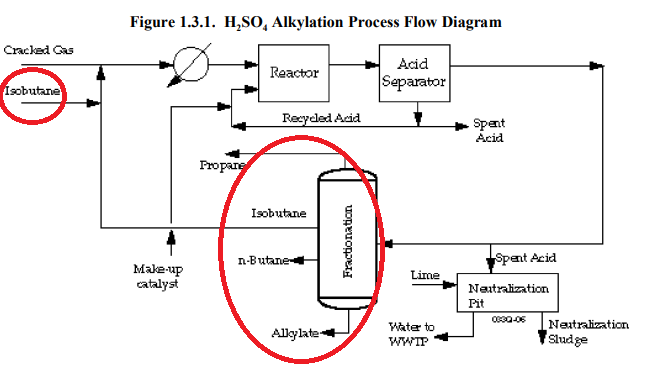
I may be interpreting the information provided within the EPA study incorrectly, but it appears as though high purity n-butane and propane are made by using Isobutane as part of the process…
Yes I understand it’s a natural gas, I totally worded that incorrect. But what you are completely missing is, Isobutane is obtained by isomerization of butane. I keep saying this and you keep insinuating that I’m on drugs?
I think he may have a differing opinion, as do others who have spoken.
Its quite literally an opinionated discussion and I truly appreciate your contributions but part of the reason I made this discussion was to separate this “rhetoric” from any other established discussions such as TheLostBiologist’s hydrogen peroxide thread… mainly to save you and others the annoyance really… if you want to participate that would be great but knit picking over someone’s spelling is going to echo chamber my thread and my ability to gain knowledge about the subject matter.
Would like it if we could keep the conversations going in a positive direction.
o thought they just fractionally distill it based on pressures?
If you guys want me to shut up, then show an unbiased and transparent experiment of using pure isobutane to grow diamonds. Can you grow diamonds from it? Yes. Will they be easy to do in a jar? Not at a wet saturation level it won’t. Will it be easy in a miner? Maybe.
My observation using pure iso for that last year has been that jars will not hold the pressure required to grow facets with isobutane. Jars that hold pressure, will redissolve. Jars that off-gas will drop out clouds of nucleation. Redissolving nucleation clouds in the solution are much easier than the nearly impossible feat of redissolving chalk in a jarred isobutane solution.
Isobutane solutions will jump out of a jar just like I have seen happen to many others who are fearful of their pour offs. To counter this I have taken the oil to a muffin and scraped into mason jars. Then I heat the jar to the melting point of the solution. At this point you can either temp cycle or burp into nucleation while maintaining warm temps.
Again, all of this can be observed by just one of you documenting your isobutane diamond growth in a jar. I’m not here to serve up biased conjectures. I am just sharing my observations and would like an actual scientific method to be followed before claims are made or claims are dismissed.
I have done nothing but speculate and state my opinion, only to be trolled and ridiculed by someone who holds a position of power in the industry. Someone who has been trying to sell consults on fixing the “medusa gas”. It’s ok to be wrong guys. I understand you all want to figure it out but don’t call me crazy and don’t troll me until you have proven me wrong with undisputable scientific documentation. Then troll all you want. I would even admit I was wrong!
That’s the point. Some butane is used to make iso but the rest is just distilled. Distillation does not cause isomerization, it just separates.
I’m under the impression that the instrument grade purity is manufactured using the isobutane as well, hence why it is in trace PPM in all three solvents and blends? If you look at the red circles above, that’s why I am saying that…
So you increase purity by introducing impurities?
That makes no sense.
I believe it is supposedly used as a “reproof” if you will, where as its continuously added in their refinement stream for the higher “cleaner” purity n-butane and propane…im… not 100% but I think if you read the EPA study it will say that.
EDIT: they are not introducing impurity, they are introducing the “opposite of that”. Perhaps the impurity you speak of is the catalyst used to make the isobutane?
EDIT: Ahh, there it is… this is what i am referring to…above i mentioned;
A series of fractionators distills the product streams from the reactor into the alkylate,
saturated gases, and HF acid. Isobutane and HF are recycled back into the reactor as feed.
The main fractionator overhead is charged to the depropanizer and debutanizer, where high-purity propane and butane are produced. The propane and butane are then passed through
the alumina treater for HF removal. Once catalytically defluorinated, they are KOH-treated and
sent to LPG storage.
You’re literally responding to a guy who used to grow in isobutane. I’ll go find dred’s posts from 3 years ago, if I have to, but I don’t really care that much
Blends have existed for years of butane/n/iso, and folks have been crystallizing in those for just as long. You sound like a braindead idiot for harping on something we’ve all been buying for 8 years.
Why is something people have been using as a solvent only now giving us issues? Why are there no chalk/medusa/fast crash crystals even two years ago?
Answer that lol
Nice try bud
What’s your favorite flavor of crayon?
Just go crawl back in your hole. You’re now blocked
This is the first time you’ve given an educated explanation of why you think it’s isobutane. Everything else has been single sentences or memes.
Once I started reading this thread I realized nobody understood what I was actually suggesting. I have had many private conversations with people on here about what is going on with my experiences using iso. I don’t owe anyone on this forum an explanation. Just because I have said "lol isobutane " or posted a meme does not mean I have no explanation. I have dozens of photos and videos of jars I’ve poured with isobutane. I don’t care to help but I will defend myself with an educated answer when the trolls try to stifle my reputation and attempt to bully me. I will not be silenced and I will not publish unpaid research for a bunch of haters.
Sure as fuck seemed like you had no explanation. You would intentionally troll the group with your bull shit “lol isobutane”
The only reason you’re saying “lol” is to troll people like @Dred_pirate.
You’ve been trolling the entire time by not giving an explanation to your theory. We’re not asking for your information, but if you’re going to suggest something, at least be here to explain your reasoning. Now your upset that you got trolled!?
This is next level trolling on your part man and you fucking know it
Aren’t you guys all licensed extractors? Why not just buy a tank and prove me wrong. I’m pretty sure someone did buy a tank and immediately got fast crash every run. Then all of a sudden the goal post was moved because “what if the isobutane is also contaminated”.
Just buy a tank and document the process with real scientific method. You know, maybe a control group and some data. Pretty easy to prove me wrong if I’m wrong, right?
I don’t care to because I don’t agree with your theory. I like to have level headed conversations on ideas like yours. What’s wrong with just explaining yourself instead of complaining about people trolling you?
