That doesn’t explain the chalky stones though does it?
Milly/chalky diamonds make me immediately think of dissolved oxygen or water…
2C4H10(g) + 13O2(g) → 8CO2(g) + 10H2O(g)
This actually just made me think reallllllll hard. As these are by products of combustion… Almost ever reaction I see also looks like carbon dioxide off gassing. Not butane or another hydrocarbon.
A milky solution full of chalk, or a “snowglobe” as I’ve referred to it is just a cold solution precipitating rapidly in my opinion.
A milky diamond could be explained by waxplugs comment “My take on what’s happening is that just like in water, when you freeze cold water you get air bubbles trapped in the ice that make it cloudy. If you heat the water first, the air rises out of the water and it produces clear ice when it freezes”
And lastly the chalking diamond in my opinion Is a result of an isobutane inclusion finding its way out once removed from solution
If you examine this quote, will Billy posits that they would not have this problem occur ever due to his procedure. [quote=“TheWillBilly, post:175, topic:168464”]
We dont even have the issue is his point and anyone processing like us wouldn’t have this issue
[/quote]
I would guess that he is suggesting that they are recrystallizing in a high BP solvent such as limonene or heptane as these solvents stay fairly “warm” through evaporation, keeping the lattice free of solvent inclusions.
DISCLAIMER: This is my theory, remember to be nice!
Lower your temperatures to near the boiling point of your solvent stop being like 50+ degrees off, you have a thick oil preventing the escape of your gas, that is were the air bubbles are coming from and why the product is foaming. ITS A GAS UNDER A RESIN. WHat do you expect something so volatile to do when trapped under something with a much higher boiling point. ITS TRYING TO ESCAPE. Go to Graywolfs site and read on thick and thin film purging maybe that 8 year old information may point you somewhere positive.
Why does gas that boils around 0 degrees F avg boil so quickly at room temp. hm…
Retracted cause im being a troll
@hambread you’re correct, I would argue the same thing and I had the same thought, I showed it to our tech and he said pretty much exactly what you said.
Still, I’ve always been defiant. I must learn for myself. It looks like Ammonium Bicarbamate could be the potential focus here? I was mistaken, in that; DEA and MDEA do not “isomerize” or turn into NH3 though I’m not sure if I said that verbatim in my postings above, its possible I did, but definitely what I guess I was thinking initially, and turns out the MDEA was just a clue. Its the way the MDEA and DEA chemical solvents carry over the NaOH which becomes mixed with ammonium bicarbonate granules and some how “leaches” some kind of trace back into the output stream.
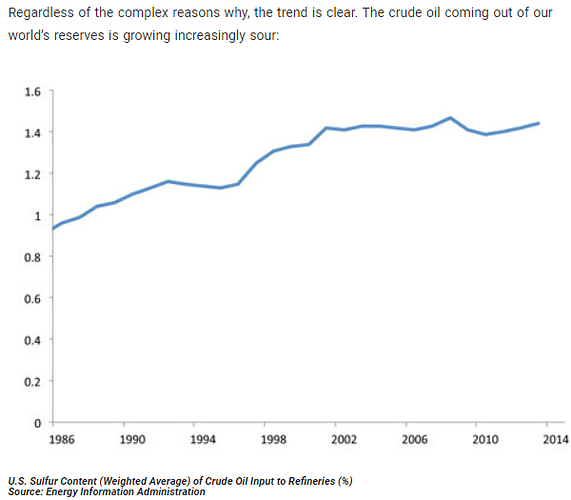
It seems like the ONLY thing now is finding out exactly how sour is the crude? If they used sweeter crudes prior to the global pandemic and changed over or added on sour crudes then you would see this affect, possibly, and be able to determine “its the gas”.
I wanted to find something conclusive but just couldn’t do it; there are market reports behind paywalls regarding MDEA and no doubt contain the information I would need to draw a correlation but I had to stick with public access information.
Here’s what I found below;
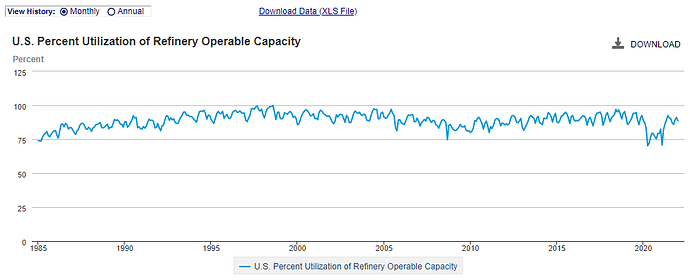
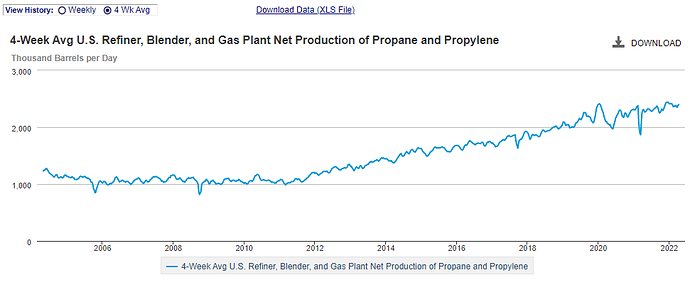
EIA statistical information; paints a picture of slump in operable capacity at the refineries but an uptick in supply of our product segment. (the chart says Propane/Propylene but it referes to ASTM D1835-20 standards which includes all the LPG’s)
So you mean to tell me output product increased while operable capacity went down or remained stagnant? HOW…
original link here
Here’s the paywall report on MDEA global markets outlook 2022;
https://www.precisionreports.co/TOC/19646475#TOC
We’ll have to make do with this instead; original link
WASHINGTON, Nov. 16, 2021 (GLOBE NEWSWIRE) – According to a new report by Vantage Market Research, the n-methyldiethanolamine (MDEA) market is expected to reach USD 1000.2 Million by 2028, growing at a CAGR of 15.1% from 2021 to 2028. The increasing demand for carbon capture and sequestration (CCS) coupled with demand from oil & gas industry is anticipated to fuel the growth of n-methyldiethanolamine (MDEA) market within the forecast period.
original link here
Detailed description of Merox treating process and what I’ve learned;
Caustic Soda = NaOH (Sodium Hydroxide),
Removes H2S and most but not all CO2, and requires emissions are higher in oxidizer for regeneration.
;added with;
Chemical Solvent / Catalyst used in Treating of Caustic = DEA (diethanolamine), and or MDEA (methyl diethanolamine)
Removes some H2S and all CO2 slowly, and is separated via reflux condenser for recovery. DEA offgas emission output is higher compared to MDEA with no output/off-gassing required.
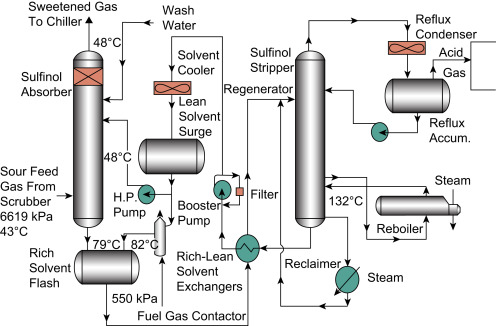
Sulfinol (a mixture of sulfolane, diisopropanolamine or methyl DEA, and water)
Regenerator IN = Rich amine solution (rich solvent) = chemical solvents with mercaptans catalytically oxidized to disulfides
Regenerator OUT = Lean amine solution (lean solvent) = recovered chemical solvents from extracted disulfides are seperated via reflux condenser
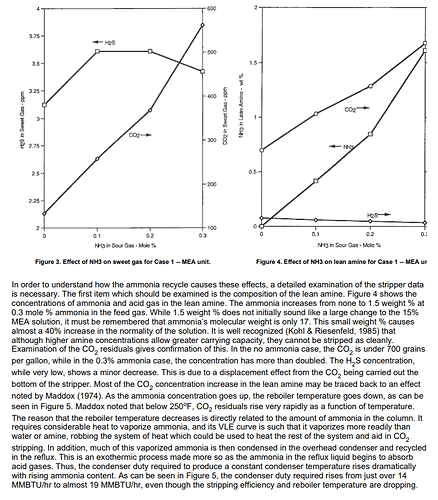
Small amounts of ammonia can cause serious problems in some amine sweetening units. These problems are usually traceable to a complex of ammonia with CO2 in the stripper. When large amounts of CO2 are present, this complex may cause a build up of CO2 and ammonia in the circulating amine. In MEA, ammonia tends to push CO2 into the reboiler, increasing the CO2 residuals and ammonia in the lean amine. In MDEA, the ammonia appears to help drive CO2 overhead, decreasing CO2 and increasing the H2S in the stripper bottoms.
CO2 capture process must be used to control the concentration of this greenhouse gas before being discharged into the atmosphere. Chemical absorption using aqueous amine solution is the most effective technique which has been used for decades for removing CO2 from many industrial sources. This technique is also suitable to reduce CO2 emission from post-combustion power plants. Amine development for the capture of CO2 has been particularly focused on blended amine solution due to its effectiveness in increasing high CO2 loading capacity and decreasing amine degradation, corrosion rate, energy consumption during regeneration, and also the overall operational cost. MDEA, one of the most popular tertiary amine used extensively, has many advantages, such as low energy consumption, low corrosion, high degradation resistance, and good absorption capacity [2]. However, its reaction with CO2 is slow, thus blending with primary and secondary amines, such as monoethanolamine (MEA) and pipe razine (PZ), is essential to increase the CO2 capture reactivity. Diamines, specifically diethylenetriamine (DETA) can also be blended with MDEA to enhance the solvent kinetics and absorption ability as confirmed already in our previous study [3]. Fig. 1 shows the chemical structure of DETA and MDEA
CO2 + NH3 = Ammonium Bicarbamate (occurs in atmospheric pressures @ 0c)
Ammonium Bicarbamate can be formed by the reaction of ammonia NH3 with carbon dioxide CO2, and will slowly decompose to those gases at ordinary temperatures and pressures.
Other things thats could output in theory due to amine treating conditions;
- carbamate ions
- ammonium carbamate
- ammonium carbonate
- ammonium hydroxide
- ammonium bicarbonate
- calcium bicarbonate
- urea
CONCLUDE:
Just seems like the CO2 and Ammonia from sour crude could be combining in the Merox treatment (sweetening process) to create Ammonium Bicarbamate which carries over in combination with the treated caustic soda and is exposed to the output LPG stream. Likely minute(myne-oot) enough that its a non-issue but possibly due to increase of MDEA used to maintain emissions, the problem is compounded and you have trace residuals that are maybe N.D. but noticeable into output?
More LPG makes more H2S which requires more NaOH to treat caustic, which results in larger build up of CO2, which carries over Ammonia and pushes the two compounds combined as Ammonium Bicarbamate into the reboiler which then recirculates into the “lean amine solution” aka lean solvent (clean solvent) which directly contacts the output stream “sweetened” product when set back to treating process.
Fun fact they say the process is called “sweetening” from “sour crude” but you’re removing salts so think of it like your sweetening the product to remove the saltyness.
I still claim nothing, I am not conclusively indicating that THIS is it, but appreciate the opportunity to share my thoughts.
A problem I am having with this is; the sour feed temp is supposedly 43c, then taken up to 79c in the solvent flash, then into the reboiler after its been regenerated, reboiler at 132c… but it goes into a Reflux condenser, and I don’t know what temperature… I’ll dig around I think its in one of the papers…
If this is the case, then I assume there would be no ammonium bicarbamate? it would just be CO2 and traces of Ammonia gas from sour crude. If this is the whats up, then I guess my theory is; more sour LPG meant more residual Ammonia from the merox process and it just built up excessively more than usual within the supply from the last two years. It should be a quality control factor though and assume they would find too much NH3, and reject for supply?
Otherwise; can, or how does, the Ammonium Bicarbamate degrade back into gas? Its a solid, so it would show up in the solvent, or there must be a point in time where it would degrade back in to CO2 and NH3 gas? Unlikely?
Are we “making” ammonium bicarbamate in the solvent due to condensing/cooling temperature setpoints? Is it reacting with the THCA?
EDIT: Hey, just found this! looks cool, please read?
Effect of Heat Stable Salts.pdf (410.2 KB)
In a recent article dealing with a live steam-injected stripper (PTQ, Q3 2012), we discussed how steam usage affected the stripped water residual ammonia and hydrogen sulphide levels, how ammonia distributed itself within the stripper in an unexpected way, and how Murphree (1925) vapour efficiencies varied with location within the tower at various stripping steam rates. The discussion was limited to non-phenolic sour water. In the present article, we examine the effect of heat stable salts (HSS) on sour water stripper (SWS) performance, how the injection of caustic soda can spring ammonia from the sour water, and how caustic injection can worsen H2S stripping if it is injected at the wrong place, or too much caustic is injected. The analysis uses a mass transfer rate-based simulation model for sour water stripping and for assessing the distribution of ammonia in conventional amine treating systems.
it goes on to say;
it may be useful to point out that ammonia and hydrogen sulphide have almost unlimited solubility in water when they are present together. This is an interesting consequence of the fact that the reactive component of the solvent, ammonia, is volatile and, if present in the gas phase, it will continue to absorb as long as it becomes protonated as a result of H2S co-absorption. Thus, it is conceivable that a particular sour water stream may be a lot more concentrated than the solubility of either ammonia or H2S by itself might suggest.
and;
Here is an email I just received from Jack McCulloch, the director of hydrocarbons at gas innovation
Any answer was to why my GI stainless tanks through kushco gave myself and a shit load of others benzene?
The last 2 tanks i have gotten in the last month from gas innovations is showing the chalk insanely fast
Any thoughts on my observation that I poured same run into 2 jars.1st was smooth pour. 2nd was a scrape out and then the scrape out one crashed and when i opened was bubbling diamonds. the smooth pour was honey with a tiny few diamonds starting on the bottom. same run.
The scrape got more agitated, less butane, the pour had more, saturation levels were different,.
that’s how you get different consistency, the pour jar will take longer as it had no agitation to drive out the butane, but the scrape will crash fast.
Maybe ask them if the “molecular sieve” beds are highlighted or delineated somewhere in a process flow PFID, and ask them who does that part?
I’m currently not aware of any bead beds being passed through, surely I have not seen it in the refinery process, if this is true, it means we shouldn’t see any issues? Be careful if any of those e-mails are confidential.
Hi Boss,
I’m not fully confident just yet as I am still trying to do the research but it looks like Diversified uses ASTM D-6667 as standard for checking for Sulfur. I’m not sure, what ASTM standard is used to check for Benzene yet but you would think ASTM D-2163 would be it, and I BELIEVE the refineries adhere to such standard but I haven’t found anything verbatim telling me that they analyze to ASTM D-2163 standard?
Will update more in regards to the testing standards once I find more definitive information.
…we leave that for the end user…
And 13x +Activated Alumina does seem to do the trick.
Did a side by side the other day
Oil wet with residual iso, added n butane and capped
Oil that has been purged at 115 f overnight, added n butane and capped.
Results:
The isobutane residual jar was opened at room temp of 78f. The jar sat still for 5 seconds and began to violently boil out like a rocket. I capped to save it. Continued with each jar from batch same result.
Nbutane jars were opened with zero violent boil out. Some agitation creates boiling but nothing that like the other jars.
The iso jar is all chalk
I think the reason you keep insisting it is iso is your definition of chalk is different than the people experiencing the issue.
All the diamonds from Medusa gas turn from clear facets to this chalk. This is chalk, rapidly precipitated micro THCa is a symptom but not the tell tale one. You can rapidly precipitated from almost any solvent. The strange thing about the “fast crash” rapid precipitation is they are shaped like bars and turn to chalk after seperation. Not while still in the jar.