I know activated alumina is used for sweetening, and you can remove the mercaptans by warming the solvent and running it thru, pretty much, a crc with actuated alumina
I guess that the ammonia way is drastically cheaper though
I… think… I have an idea of how… just seems like its the ONLY way, might be a stretch but here goes:
Spent the whole day wondering how to connect the dots, only thing that came up is this;
The Merox treating process at the gas plants would have traditionally always been using a combination of chemical solvents. DEA + MDEA are the two most commonly used, and the most plausible scenario is that due to COVID, shut plants, labor shortages, etc, they needed to make more with less, meaning likely they would have either gone over any previously established emission thresholds, or they would have needed to add more consumables to keep up with the throughput demand…
So, you have less plants working, but you have a sudden uptick in demand. You would have to output more at the facilities that were operating, meaning you might have to look at if you are still under the emissions cap of the facility for caustic treatment. If you’re over, you would need to add more MDEA specifically, but there is a problem with adding more MDEA…remember that this lowers the amount of caustic required with the DEA, so, basically you have a secondary and tertiary alkanolamine, and a caustic soda all as sort of “one” process in the Merox treating, BUT, the problem is; more MDEA can correlate to increase/introduction of ammonia into the output stream, and its happened before!
Ammonia is sometimes present in sour gas streams which must be sweetened such as selected refinery gases, coke oven gases and reactor outlets in tail gas cleanup units (TGCU). Whatever its source, ammonia often has a major impact on the operation of amine sweetening units treating these gases.
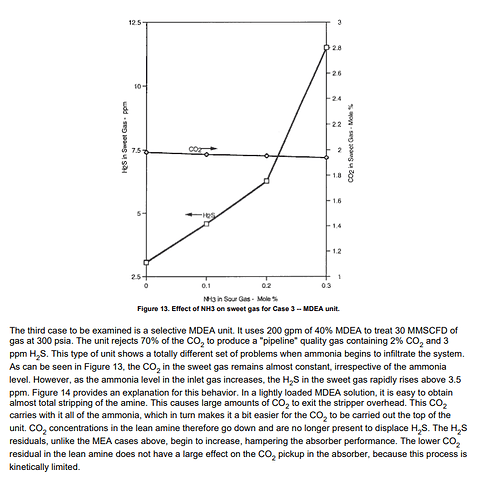
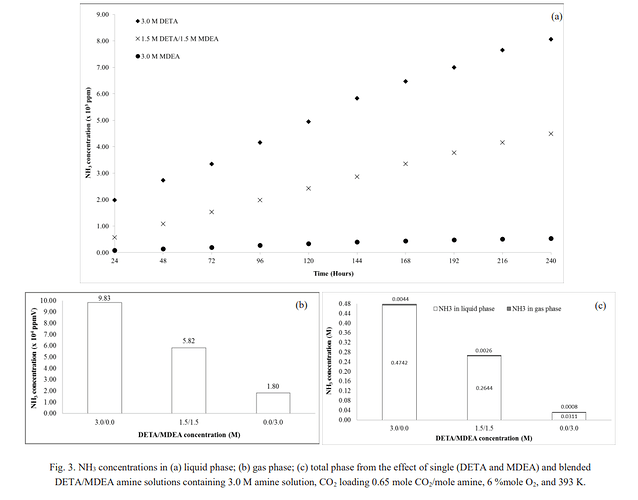
As another reference; this graph shows with MDEA and DETA, and looks like DETA is the worst when it comes to dealing with ammonia in Merox treating.
US-Refineries-2021.xlsx (10.3 KB)
So I made a list of ALL of the suspects you can see here ^^^ each of these facilities has an isobutanizer.
I took the list and searched the names one by one, and now have a bunch of news article links confirming the theme, that capacity was reduced, layoffs happened gallore, even some significant lawsuits and settlements! Too many to list off here, here is a general one.
Can see them big oil gas pockets tryna get free.
It resembles closely the behavior of the amine solution foaming
do you see the light fraction analysis posted above right out of the ground at the Bakken fields?
This? Can you reference the original?
Google Bakken crude composition.
Not all crude is the same.
I read it as butane is feed for isomerization process?
Of course unreacted butane is a output…
You are correct, likely we could have started using more sour crudes recently.
The Merox process sweetens the LPG (butane, propane, isobutane) from sour crudes to remove mercaptans, an uptick in supply demand would require excess caustic, so additional MDEA would be needed to keep emissions low but would cause more amine carryover in general to the output stream, foaming is indicative of amine carryover, as seen in the photos posted.
I will say mine was foaming actively.
Damn that’s grate
Made my morning a lot better
I’m only human and don’t know everything but these two things look curiously similar…


and;

The residuals/charts/numbers are here;
They would have needed to use much more methyl diethanolamine since late 20, early 21 probably to stay under emission limits while working less plants.
Looks like it to me
As I commented on Cat’s ig post about your poetry, @cyclopath:
![]() So poignant are these rhymes to me
So poignant are these rhymes to me
that words elude, ergo: dots three… ![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
Magically, I also snagged ![]() #42 !!!
#42 !!!
@SafeLeaf.ca However most mentions of foaming I see are due to liquid hydrocarbon presence in the amine solution. So are we guessing that the MDEA impurity would increase the surface tension? Because it should increase the boiling point of the LPG unless I am missing something.
I could argue that “foaming” is also a result of having butane impurity in a cannabis oil . . . .
As much as I like your thought process, this is a piece I can’t wrap my head around. Would love to hear your thoughts. Is the idea that the residual amine could be reacting with a chemical in the cannabis oil?
https://www.sciencedirect.com/science/article/pii/S1876610217315151
Thoughts: I’m out of those for the time being… haha
Edit; I can’t remember where I read it anymore since I’ve looked at 20+ papers, but Ammonium salts and Sodium Acetate come up
Ammonium salts are ionic compounds with the formula (R)4N+A-, where R is hydrogen, alkyl or aryl groups and A is an anion. When R is alkyl or aryl then they are referred to as quaternary ammonium salts. The quaternary ammonium cations are permanently charged, independent of the pH of their solution.
I’m most perplexed as to how it causes this but leaves no trace, doesn’t seem to effect distillate, no isomerized thc, except my friend did just have some come back at 60%thc but 94% total cannabinoids, our labs don’t test for d10 but they said it wasn’t d8…
They made an assumption his grower sprayed powdered sulfur to late in Flower which is possible.
As the resin off gasses, it expands and freezes.