EDIT; will post more about the finding soon.
LPG stands for Liquefied Petroleum Gas and is generally considered to refer to propane, butane and iso butane. These gases are LpG because they liquefy under their own vapor pressure. Except for neopentane, all heavier hydrocarbons are considered liquid hydrocarbons. {Pentane, hexane, heptane octane, etc)
Isobutane has always been the primary contaminate I’m normal butane because it has a similar vapor pressure. It isn’t manufactured from butane and added back in.
I googled my dispensary and they have tons of this shit- just accept it. are the customers upset???
isobutane is an isomer of n-butane and still in n-butane because of the similar vapour pressure, i don’t think he @safeleaf is saying it is added back in? its a by-product of the isomerization process and some is always present in n-butane because of this. the catalyst used for the isomerization process, from what I am understanding here is what may be causing us these issues. Correct me if I’m wrong here though my understanding of that side of things is limited. it does seem to be the most plausible scenario, this would explain how it has affected so many across the continent within one year.
Science

Found this paper while looking into other industrial uses for butane and isobutane…
Seems like iso is used in blends of various refrigerants. The vaccine rollout and need to keep doses at subzero temps could def have effects on the supply chain.
Isobutane is an isomer of butane, but a naturally occurring one. It exists in the mix they pump up and is separated by distillation.
Additionally some places isomerize n-butane to isobutane to sell.


Have a good morning!
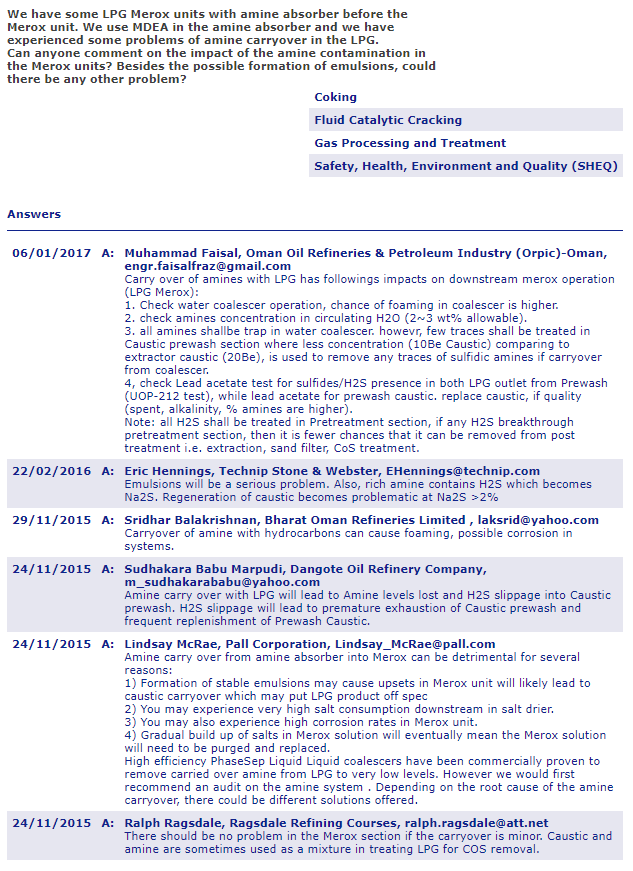
The sweetening and extraction process both use amines and caustic soda (NaOH), and there can be something called amine carryover from the caustic, one of the predominate side effects would be “foaming amine solution” which I google image searched…you should too

This is a VERY interesting conversation back in 2017, indicating this has been a problem in the past… amine carryover “into caustic?”
The other quote posted previously talks about more mercaptans since 2019, “it doubled” he said… and they were having amine carryover issues since the beginning of time, not to mention these plants can actually be “poorly” designed, and have a myriad of working issues, they aren’t perfectly maintained under quality control.
Break down of info:
The Merox process is actually a family of processes used to manage mercaptan sulfur in many different hydrocarbon streams. The process efficiency and economy have made it the most widely used mercaptan sulfur control technology in the world. It has been in commercial use in the Refining, Petrochemical and Natural Gas industries for more than 40 years. In that time over 1600 Merox units have been put into operation.
The applications can be broadly divided into either the sweetening or extraction process;
In the sweetening applications the mercaptan is converted to a disulfide, with the disulfide remaining in the hydrocarbon stream.
In the extraction application the mercaptan is removed and separated from the hydrocarbon stream before being converted to disulfide.
About 30 to 40% of the Merox units that have been put into service are for the removal of mercaptan sulfur, i.e. extraction. These applications are generally on light hydrocarbons, such as LPG, but there are also commercial applications on gasoline range materials. The process involves two distinct steps; first the extraction and separation of the mercaptan sulfur and second, catalytic conversion of the mercaptan sulfur to disulfides.
More about “extraction” process;
Depending on the hydrocarbon source, LPG streams will have a vastly different distribution and concentration of mercaptan molecules, which require more rigorous removal methods. Some contaminants, such as H2S, CO2, and mercaptan (RSH), can be removed from the LPG stream by caustic, referred to as extraction. Other contaminants, including COS, CS2 or dimethyl sulfide (DMS), cannot be extracted from the hydrocarbon with caustic alone.
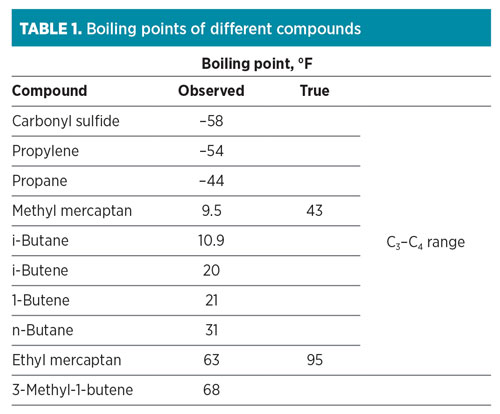
Caustic treatment (aka extraction) remains the most economical process when removing mercaptans. Fractionation, which occurs prior to caustic treatment, cannot remove mercaptans efficiently from the hydrocarbon cuts because they form azeotropes with the hydrocarbon.
TABLE 1 shows that mercaptans have higher boiling points than the hydrocarbon compounds with which they boil. As a result, the mercaptans will distribute throughout various lighter hydrocarbon fractions. Since the strong base, sodium hydroxide (NaOH or caustic), chemically reacts to remove acidic molecules H2S, CO2 and mercaptans, it can be used to remove the mercaptans or convert them, depending on the mercaptan type.
Typical and atypical impurities, and effect on design. Several common impurities, such as CO2, COS, H2S and RSR, can exist in the feed to the systems. Although it is expected that entire-stream amine treating and fractionating of the ethane cut would remove all the CO2 in the C3 and C4 cuts, CO2 has been observed in these cuts at midstream facilities to the concentration of 100 wppm–1,000 wppm. Caustic can be used to completely remove CO2 and H2S, but it is consumed irreversibly and would represent an operating cost in the range of millions of dollars.
The preferred method of treatment at these high concentrations is an amine absorber to reduce the concentration to less than 25 wppm, followed by a caustic prewash upstream of the caustic treater. COS can be removed to less than 1 wppm by pretreatment in a proprietary prewash design,a or during post-treatment with a modified caustic blend.b
Even though the typical impurities are present, atypical impurities can affect the design of a complex. These atypical impurities include oxygen, methanol, glycol, corrosion inhibitors, naphthenic acids and unusual mercaptan distributions. Various corrosion inhibitors and slip enhancers will initiate and stabilize emulsions between hydrocarbons and caustic. In addition, methanol can come from many upstream sources (e.g., dehydrators) and form emulsions with caustic. Excessive methanol quantities should be removed with a water wash prior to amine or caustic treating. Additionally, shared regeneration sections, discussed later in this article, transfer methanol from one segregated stream to another.
Glycol and other oxygenates also must be considered. These impurities can cause acid corrosion in sweetening units. Two options exist to minimize the occurrence of acid corrosion.c The first option is to construct the existing reactors from 316L stainless steel. The second option is to apply special epoxy to the carbon steel and concrete surfaces. This creates an epoxy layer to protect the carbon steel of the reactor. However, the epoxy will need to be replaced at every turnaround of the unit.
More quotes;
Relevant Properties of Mercaptans
Mercaptans are both hydrocarbons and acid gases, and they have the properties of both classes of
compounds. As we will show, the relative importance of these two aspects governs much of mercaptan behavior.Acidity
Mercaptans are weak acids relative to the traditional acid gases H2S and CO2. The C1 through nC4 mercaptans have essentially the same acidity, with a pKa of 10.6 at 25°C[1]. This is considerably less acidic than H2S which has a pKa of 7.0 at 25°C[1] for its first dissociation reaction.Acidity is important because the relative acidity of dissolved gases has a strong impact on equilibrium behavior between the liquid and vapor phases. This effect is described in Figure 1 which shows an illustration of vapor liquid equilibrium for H2S and methyl mercaptan in water. The molecular form of H2S reaches equilibrium across the vapor-liquid interface, but some of the aqueous molecular H2S dissociates into ionic species to an extent controlled by reaction equilibrium. Methyl mercaptan participates in analogous equilibria. Note that both the H2S dissociation reaction and the methyl mercaptan dissociation reaction produce an H+aq ion. H2S is a stronger acid, so it dissociates to a greater extent than methyl mercaptan, producing an excess of H+ ions. The larger concentration of H+ ions pushes the methyl mercaptan dissociation equilibrium toward the molecular form of methyl mercaptan.
Having a larger fraction of the aqueous methyl mercaptan in molecular form shifts its vapor liquid equilibrium. The net effect is that the stronger acid (H2S) generates a significant concentration of the common ion H+ and this pushes the weaker acid (MeSH) out of the liquid phase.
Moar;
Chemical Treating, Merox Extraction vs Sweetening
In petroleum refining, chemical treating is used to remove or change the undesirable properties associated with sulfur, nitrogen, or oxygen compound contaminates in petroleum products. Chemical treating is accomplished by either extraction or oxidation (also known as sweetening), depending upon the product. Extraction is used to remove sulfur from the very light petroleum fractions, such as propane/propylene (PP) and butane/butylene (BB). Sweetening, though, is more effective on gasoline and middle distillate products.A typical extraction process is “Merox” extraction. Merox extraction is used to remove mercaptans (organic sulfur compounds) from P/P and B/B streams. P/P streams may undergo amine treating before the Merox extraction to remove excess H2S which tends to fractionate with P/P and interferes with the Merox process.
- A caustic prewash of the P/P and B/B removes any remaining trace H2S prior to Merox extraction.
- The P/P and B/B streams are passed up through the trays of an extraction tower.
- Caustic solution flowing down the extraction tower absorbs mercaptan from the P/P and BB streams.
- The rich caustic is then regenerated by oxidizing the mercaptans to disulfide in the presence of aqueous Merox catalyst and the lean caustic recirculated to the extraction tower.
- The disulfide is insoluble in the caustic and can be separated.
- Oxidation or “sweetening” is used on gasoline and distillate fractions.
A common oxidation process is also a Merox process that uses a solid catalyst bed.
- Air and a minimum amount of alkaline caustic (“mini-alky” operation) is injected into the hydrocarbon stream.
- As the hydrocarbon passes through the Merox catalyst bed, sulfur mercaptans are oxidized to disulfide.
In the sweetening Merox process, the caustic is not regenerated. The disulfide can remain with the gasoline product, since it does not possess the objectionable odor properties of mercaptans; hence, the product has been “sweetened” in the extraction process, a waste oily disulfide stream leaves the separator. Air emissions arise from fugitive hydrocarbons and the process vents on the separator which may contain disulfides.
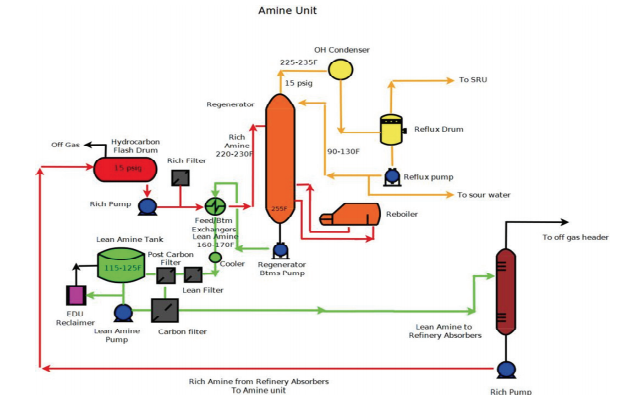
The Amine Unit Continued
Sulfur Unit | » Sulfur Recovery: The Big Picture– Part IVAmine Unit
To better understand the relationship between Amine Processing, flow and equipment, we will “walk” through the unit. Every Amine unit will be set up uniquely, but it will have most of the same basic equipment. Let us begin:
Amines and or Alkanolamines
DEA - Di’ethanol’amine
MDEA - Methyl di’ethanol’amine
MEA - Mono’di’ethanol’amine
Others but I don’t care about them…
and heres the skinny on them I guess;
When comparing the results of the simulated removal processes for the DEA and MDEA solutions, it can be seen that DEA is the less costly and more effective removal solution:
- DEA results:
- Removal of H2S from sweet gas = 100%, and removal of H2S and CO2 = 99.07%
- Average price of DEA chemical = 609,792 LE (Egyptian pounds)/d
- Total mass flow required for DEA = 794 kg/hr.
- MDEA results:
- Removal of H2S from sweet gas = 99.95%, and removal of H2S and CO2 = 68.95%
- Average price of MDEA chemical = 1,207,296 LE (Egyptian pounds)/d
- Total mass flow required for DEA = 786 kg/hr.
The simulation results show that both DEA and MDEA are effective sweetening agents for acid gas. DEA provides the cheapest and most efficient treatment option overall, with H2S removal of 100% and H2S and CO2 removal of 99.07%.
By contrast, H2S removal with MDEA is 99.95%, and removal of both H2S and CO2 is 68.85%. MDEA is both more expensive and more environmentally friendly than DEA, and it removes H2S faster. However, initial CO2 removal with MDEA did not reach acceptable levels, and it was necessary to add more MDEA and water to adequately sweeten the gas.
The gist is: MDEA (meythl diethanolamine)(tertiary) costs way more then DEA but converts mercaptans to sulfides in the stream, and separates later, (but probably not completely) so caustic waste is diminished? Also note that this is the move if you want to “improve carbon emission credits”. Then there is DEA (diethanolamine)(secondary) which causes the NaOH to be spent, so they need to replace it as the process is “irreversible”?
Couple things I want to clear up;
Just so everyone is aware and doesn’t past judgement on my part; I did not assume Cyclopath was referencing that Ammonia is the problem and that it is in the gas, rather that Ammonia can come from many places including the fertilizer or cleaning agents, but its also in the gas process apparently?
I still have no direct correlation, yet? but this is also great info I wanted to try and format to disseminate for the community.
If it’s iso, we could just take a tank of gas that doesn’t medusa and add a little iso and see it happen. Anyone have some clean gas want to try?
I do, I have a solvent tank that’s half full still from before we started to experience this. I’ll set up a time after the weekend I can try this.
There’s already 2,000 - 3,000 ppm isobutane in it. That’s typical of the 99.5 butane. How much you gonna add?
Forgive me if this has been asked but are people observing way faster recovery times with this issue or is only after pouring?
Same recovery rates as before. Many would notice the significant difference in recovery times, as well.
how does one water wash butane? do you mean “washing” like in the chemistry sense Washing a solvent? - Chemistry Stack Exchange or am i being too literal and you are bubbling gas through water?
I understand passing liquid solvent over 13x.
sorry for the spoon request
Assuming butane and water at room temp and ~30 psi you have two mostly immiscible liquids.
Stirred or shaken they will separate.
Then dry the hydrocarbon.
13x recommended.
Not my response. Sounds like a lot of work. But it is a solution I have heard, and it should solve “NH3”