I know… I know… sigh
This is a scientific forum we should all stop using Fahrenheit.
It’s a disservice to the discussions.
its definitely viewed as a feature if you aren’t doing diamonds, or until it starts crashing out in the system, then its a nuisance.
they start arguing about nonsense when we are all just trying to get to the bottom of it lol
This blog is actually VERY interesting, thank you.
if you can’t fix the bug, define it as a FEATURE…
not $#@^&(&@) sure I like the boss beating me to the punch quite so often.
![]()
although it sure as shit beats the ones that look at me like I’m from fucking mars when I suggest things.
![]()
![]()
![]()
i was thinking the same thing lol
I have a very unorthodox way to share information when the point isn’t being understood. But, I am glad to shine some light, in midst of the shenanigans
Some chalk tests out at 99% plus…reports on this forum.
Question: does extensive drying of Medusa cause transformation to chalk appearance?
What is COA of Medusa.
As far a separation chemistry…if you find 99 plus percent purity-product precipitating in the bottom of your extraction rig…you need to concentrate on enhancing this process.
A friend mentioned filtering, pouring off into a separate column. .overpressurized to 300psi in cold and getting thca plug at bottom and terps on top.
Ie forget the jars… go for high pressure stainless.
I know this is not “diamond tek”…but a 99% purity
Bang…out of column…first step is something to think about for other applications of product.
Diamonds are “Weedo-Bling” but they pay the bills…and this forum is all about making money for every one.
100% , you can just recrash in pentane to get that bling diamond look after the fact anyways right
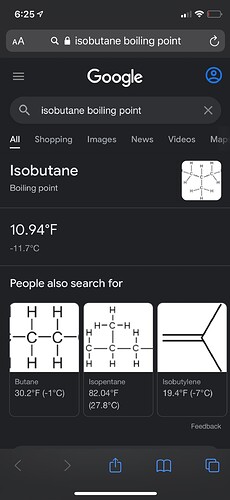
I didnt bother explaining my reply i was referring to the boiling point of 70-30 mix. Im aware of the boiling points of individual solvents engineering toolbox is amore than reliable source for so.
@kromatik1 100%
Thats the strat
Here’s a few quotes to stir the pot. Hope you guys are having a good day! Remember to be nice
If you really dig into the butene/iso-butane separation process and what goes into it you find alot of interesting shit that could lead here to our problem. My curiosity is, has demand picked up so much that these processors are willing to send 2% trace amounts of “higher purity” “butane” back into the supply chain because the margins on the iso are so much higher, that the loss on an acceptable/superior (iso tainted) “dirty gas” is no big deal.
Are elons demands for rocket fuel that high?
I’d think they’d run the gas in a closed loop over the catalyst until they reached desired yield, separate the iso from the n-butane and send the n-butane back to the catalyst rather than try to sell the n-butane that could still be mixed with more profitable iso (as I said previously although maybe not as clearly).
I’m just saying we all know there is a point of diminishing returns, where it becomes easier to move it forward than try again. I’ve also seen intentional loss’s balanced by large gains more times than I can count. We have all sacrificed effecency for volume at some point.
Did some more digging, found something interesting but haven’t fully drawn the potential correlation… still interesting concept; bare with me…
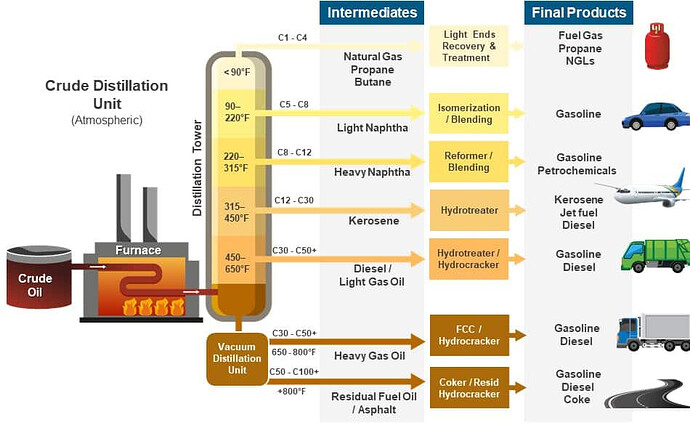
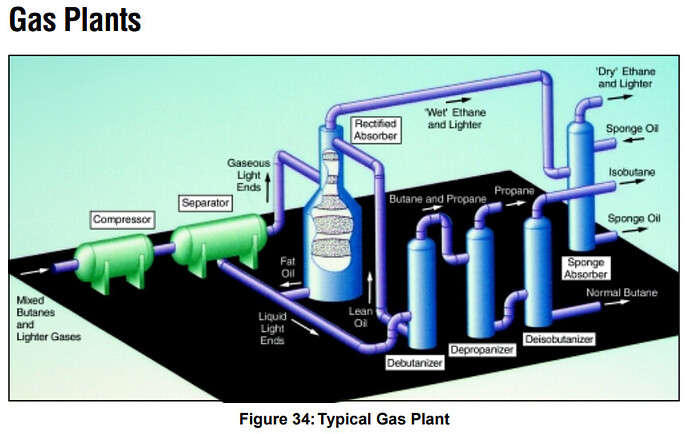
I like this chart, shows you temps, the naming of C1-50-100+, categories and labels…
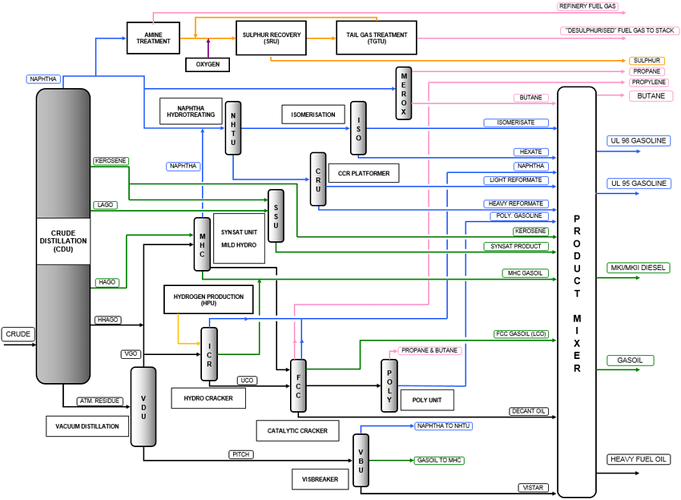
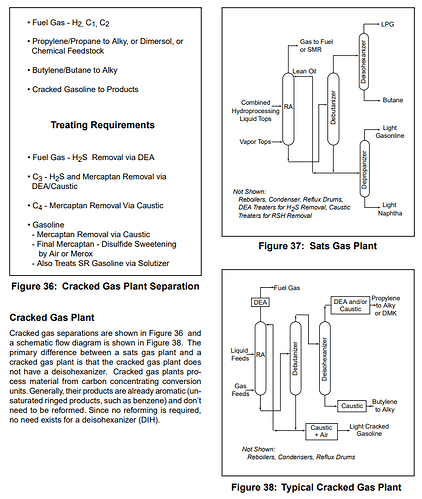
Here is a more detailed version of the process flow I’ve posted previously, check out the “Merox” unit
Not sure if you can see it but its here as well, and directly outputs LPG and Butanes, I think this is the best process flow i’ve seen, it seems detailed enough.
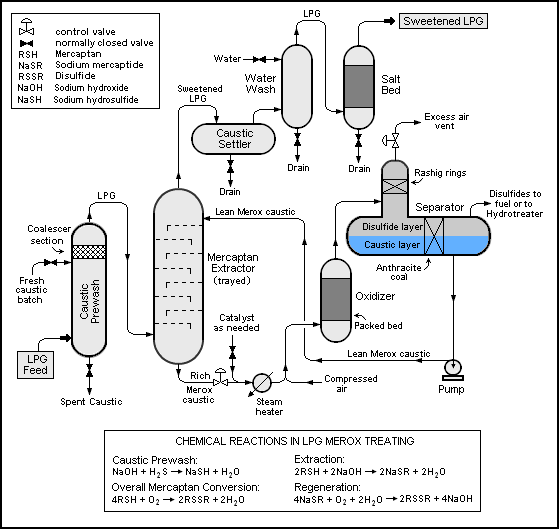
What is Merox treating?
Its a way for them to remove Mercaptan, H2S, and CO2, but there are several ways to skin a cat and I’m wondering if how they skin said cat could have changed post mid 2020? If you search Merox in Wikipedia is says this;
Merox is an acronym for mercaptan oxidation. It is a proprietary catalytic chemical process developed by UOP used in oil refineries and natural gas processing plants to remove mercaptans from LPG, propane, butanes, light naphthas, kerosene and jet fuel by converting them to liquid hydrocarbon disulfides.
The Merox process requires an alkaline environment which, in some process versions, is provided by an aqueous solution of sodium hydroxide (NaOH), a strong base, commonly referred to as caustic. In other versions of the process, the alkalinity is provided by ammonia, which is a weak base.
The catalyst in some versions of the process is a water-soluble liquid. In other versions, the catalyst is impregnated onto charcoal granules.
This process is referred to as “sweetening” and the caustic byproduct can be troublesome to deal with so it might be nice to have a “caustic-free” process… which uses Ammonia.
Medusa are you NH3?
It kind of looks that way to me.Faceted then turns to dust.
No longer in our gas we trust.Isobutane or benzene?
some suspect it’s propylene.A water wash, and 13x
No longer does the problem vexSo what causes chalking stones?
Do we have but more unknowns?No, I think we’ve found it now
And this picture tells me howPhoton_noir & Roguelab have both shown
Crystals in ammonia grown.
So, curiously I was going to see if this was even a thing, there was a reference paper from 2003 on the wikipedia page below that I could only access from the wayback time machine, kind of wierd but okay… In the paper it says;
Okay, so, are they even doing this? That is where I couldn’t progress any further because I found some info talking the process down as being expensive and requiring additional equipment. But still an interesting concept.
Ammonia may be used to remove H2S, CO2 and other acid gases such as HCN, COS, CS2 and mercaptans. However, a serious disadvantage in using ammonia for sweetening is the extreme corrosiveness of concentrated ammonia solutions which requires the use of expensive alloys in construction. In addition, ammonia sweetening processes are usually more complex than amine sweetening processes, thus requiring additional equipment
and
Small amounts of ammonia can cause large problems in amine sweetening units under certain conditions. These problems are usually traceable to a complex of ammonia with CO2. If large amounts of CO2 are present, this effect may either cause a build up of CO2 and ammonia in the circulating amine or rob the lower section of the stripper of CO2 which is needed to displace H2S in the lean amine. In MEA, and it is to be expected with DEA and DGA as well, ammonia tends to push CO2 into the reboiler, increasing the CO2 residuals and ammonia in the lean amine. In MDEA, the ammonia appears to help drive CO2 overhead, decreasing CO2 residuals and by inference increasing the H2S in the stripper bottoms. The secondary symptoms of ammonia contamination may be excessive water loss, high condenser temperature, low reboiler temperature or high acid gas residuals in the lean amine. Depending on the operational problem observed, ammonia contamination can possibly be corrected by increasing the reboiler duty, decreasing the condenser duty, or using a sour water stripper on the reflux water.
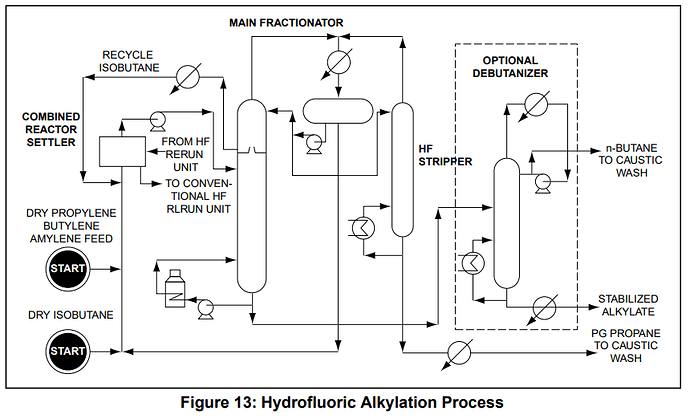
I also wanted to touch back on the isobutane real quickly, it seems inherently that it is in trace within everything and it seems to be a feedstock they are constantly reusing that does come into intimate contact with the other LPGs so however the make the isobutane should or could have an affect regardless of if the “butane and propane come off seperately”…if you look at the alkylation process, its in constant recycle and there is a “wet isobutane” and “dry isobutane”
So I am offering two possabilities;
- Maybe they used a caustic-free solution (ammonia) to remove mercaptans and it only started around the time the fast crashing started happening?
or
- Sudden demand of gas for cars, but having shut plants and labor shortages, caused the plants that were churning isobutane out to churn it out waaay more and to try and keep up with demand, meaning more alkylation, meaning more isobutane in the mix, meaning potential more residuals of catalyst processes? It also says that n-butane goes to a caustic wash as well, so something they use there could be causing it?
Update:
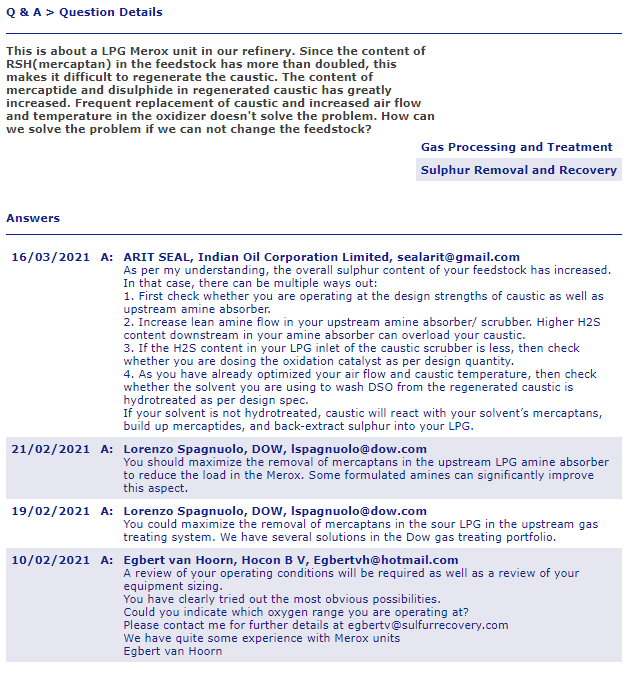
Holy crap this is exactly what I was looking for, an insiders perspective is worth its weight in gold and I’ve somehow stumbled upon a literal gold mine? Check this website out and look at the first few “questions” posted in this weird looking forum of what looks like petrochemical engineers talking shop!
http://www.eptq.com/qandakeywords.aspx?k=81188c8a-4d64-4b2f-8144-9e87a5309699