So this might feel like a stupid question at first, but the further I try and delve into it, the more complex it becomes.
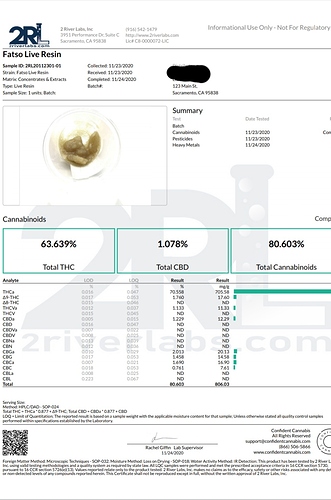
When you buy a gram of solvent-extracted product - let’s say, Live Resin, made from butane/propane - from a dispensary, it will say on the packaging “70% THC, 80% Total Cannabinoids”. What is the other 20% of the extract comprised of?
Before you jump to your first answer, it is worth mentioning that the “80% Total Cannabinoids” is only calculated using the 12-15 cannabinoids that a state-licensed testing company will search for (THC, CBD, CBG, CBC and their acid forms, CBN and CBNA, THCV and THCVA, D8 THC…maybe one or two others?). So the extract may contain several % more cannabinoids than listed, of which we do not have sufficient and sufficiently cheap tests to reliably identify them.
So other than cannabinoids, what are the major plant-based biochemical groups that are (even partially) soluble in non-polar solvents?
Terpenes? That’s a few %, especially from FF product.
Waxes? Presumably primarily from the plant’s cuticle.
Fats/lipids?
Colorants? Flavonoids (and its subgroup Anthocyanins)? Carotenoids? Chlorophyll?
What else is there? What else am I not thinking of? Obviously there is variability based on a variety of factors (e.g. genetics, grow tech, extraction tech, soak time, solvent temp, etc etc.) but there should be known groups in this field.
What we smokin, eh?