They’ll notice all of the silly names attached to the email addresses and figure it out. Haha
I have been experimenting with cycling right around those temps too. My only problem is having a vessel hold a large amount of mason jars at a steady temperature in that range while also being C1D1. Any suggestions on how you do it? I was thinking about using a circulating chiller outside my booth, with this plumbed inside the booth safely with the jars sitting inside: Temperature-Controlled Tank | Plastic Jacketed Tanks | IFP
That seems like an economical solution. I was lucky enough to walk into a 12" x 12" jacketed vessel at the lab that the previous tech left here. I am only experimenting with one jar at the moment so space for jars is not really an issue for me at the moment. Maybe we should move this conversation to Application of temperature cycling for crystal quality control during crystallization as to not clog up this thread.
If your jurisdiction requires mason jars/crystallization vessels to be kept in a C1D1 control area, perhaps you could consider jacketed diamond miners? Kind of a big investment cost there, especially if you’re running many strains, but you could easily manifold them together and temperature cycle compliantly.
Back on topic.
I hear a few theories here, but they all center around contamination of the gas, it sounds to me.
Have all affected parties in fact tested for benzene? It sounds like @johnbigoilco definitely has not, if I’m reading right.
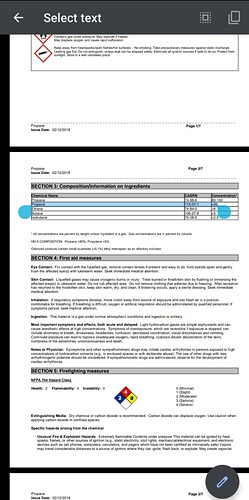
What about Propylene? Have affected parties tested for this as well? I know it’s been mentioned by a few people, and the boiling points seem to suggest to my admittedly ignorant self that it could be a contaminant.
These seem to be the best competing theses that I’ve read so far. A limited number of lots of tanks have been filled with an unknown contaminant (seemingly propylene), shipped off into the wild, and slowly working their way through the supply chain in a sort of pockmarked fashion (aka, why it seems like there are clusters/hotspots of labs located together that experience this crystallization issue.) Assuming the solvent contamination theory is what it is.
I am ignorant and can’t contribute much, but I’m watching with interest.
All tests are showing N/D for benzene. And with coa’s from the solvent supplier showing a higher amount of propylene right around when we started to see problems. Definitely says something
2nd this.
My next question, have you had your solvent tested after beginning again with fresh 13X mole sieve @dred_pirate? It sounds like for you, anecdotally, refreshing your 13x stopped your brief (?) experience with the chalk/medusa diamonds.
It seems just from Delta Absorbents description and your article, 13x grabs a lot more… types of molecules that just moisture, and if we’re lucky, propylene and or whatever is altering the crystal morphology is one of the things getting grabbed.
Just trying to summarize this thread. Appreciate ya’'ll sharing your experience with this… unexplained and hard to control result. Just being able to recognize this will be valuable, if I end up encountering it in my own work.
As soon as I have the x13, the tests shall commence. A scientist for one of the gas companies came to our lab. We showed them everything and all our data. He took samples of the pentane mother liquid from a run that had turned to chalk when it was a butane extract. After redisolve in pentane the structures formed normally. The scientist thinks the impurity will be In the mother liquid part. Also took samples of our pentane straight from the container to get a baseline for what we used.
I wish you the absolute best of luck. You’ve been incredibly forthcoming with this issue, and I’d feel privileged to try your product, medusa or not.
If your in SoCal your more then welcome to come by. I offer anyone interested in the issue the same Invite.
I have some that I’d like to get tested too. The mother liquor, structures, Medusa ones, clean gas, dirty gas. All of it.
Is this in @kcalabs purview? It feels like a strange enough result a well regarded analytical team might be helpful.
And it appears that in different applications, the 13x is used for removing propylene from isobutane and propane. They are using it differently, but I don’t see why it wouldn’t adhere to some in vapor phase. I actually noticed it mostly when I wasn’t able to get any new 13x for a short period of time, which was when all of this was happening. I ended up reusing my mol sieve and it kept happening. I recently changed them as delta adsorbents was out of stock previously. @Dukejohnson said that he was changing his out quite regularly, daily I believe. And he has had far less problems than he has had in months. I did have a jar form a small xani bar the other day. I put it in the oven and it’s gone now. And the normal structures are growing fairly quick, too
“[Screenshot_20211025-222036_Drive] lamia2007.pdf (519.8 KB)”
Is a demonstration that isobutane can act as an effective mobile desorbant
to separate propane and propylene* when x13 is the stationary phase
in a “simulated moving bed apparatus” at the specified flowrates and temperatures and particular choice of x13 particle size. Take a close look at figure 15.
Why anyone will think a simple batch adsorption proceedure is going
to separate propylene from n-butane with a system generating
“1 or 2” theoretical plates is beyond me??? i.e., their experimental
set up is much different than the simple CRC mol sieve columns used here,
and also different than in any redistillation proceedure in use.
I emphasize they are using isobutane as the desorbant! It is a difficult paper to read for most.
This is not to say, that x13 might not work as an adorption isotherm to
remove the “unknown contaminate”…it may well work, but I doubt
it is propylene that removed by any “batch process” available to this
forum.
add some propylene to the pentane and see if it chalks out!
if propylene fucks things up, it should be very easy to prove or disprove.
Why FicklePicle’s “chemist friend” thinks it is intuitively obvious
that propylene would facilitate the formation of alternative
crystalline forms of THCA -(X?) or “be problematic” is rather odd.
Unless, he just means he has prior knowledge that propylene
is a contaminate of n-butane?
If scrubbing with x13 solves everyone’s problem, it is
unlikely due to selective propylene adsorption in the presence of butane:
separating propylene from butane gas is NOT what that paper is about.
edit thanks to point by Dred_pirate:
*Propene, also known as propylene, is an unsaturated organic compound with the chemical formula {\displaystyle {\ce {CH3CH=CH2}}}
gas distributors refer to it as propylene.
I wasn’t seeing chalky diamonds when I used gas station propane and that shit is mostly propylene.
Guess not mostly but a significant portion.
might behave differently with propane instead of n-tane
Propene. Not propylene.
It actually is. Amongst others. But, usually in far lower numbers. Under 1ppm
If he’s an actual chemist, not just trapchem like we perform, there’s a good chance that he possibly read about this, or it was an assignment.
It is the only variable that shows anything
Propene and propylene are different names for the same chemical.