Hey Folks
Wanted to share data I generated by percolating MCT through a few batches of Sweet Rainbow Hemp.
Over the past week or so I have been working with @TheKid420 on home counter-current lipid infusions. He is using coconut oil so heat is absolutely necessary. In my experiments here I simply used MCT at ambient temperature.
What I’m mainly trying to show is that you can create potent infusions by either percolation or maceration if you thoughtfully stage your batches and washes. On any scale–from home growers to farmers looking to process their own biomass into an effective (full plant synergy) and potent product. So I see building awareness of infusions, with data, as a way to also build interest in the equipment my company (https://www.hempharvestinnovations.com) builds. Those interested should also see my topic on economics of lipid infusion: The Economics of Lipid Infusion
Maceration turns in to a lot of work on small scales so I put together the following percolator. It is composed of two six inch spools with a mesh gasket screen and a valve at the bottom. I like to fill it up about halfway with moderately-packed biomass and leave the upper spool empty to pour carrier oil in to. Basically the goal of the experiment was to produce baseline data on how much carrier oil is needed to sufficiently extract a batch of flower in addition to how the vial potency can be stepped up by running subsequent batches.
For potency tests I use an hplc that I have calibrated for cbda and cbd. Potency of starting and spent biomass were Soxhlet extracted with ethanol to determine potency. I only did one hplc sample per vial sample so the reported values may have a few percent uncertainty.
Experiments:
-
freeze biomass at -30degC
-
hand grind biomass and filter through a ~2mm sieve
-
place a scouring pad filter at bottom of percolator
-
pack 21 g sieved material into percolator (batch 1)
-
place ~50 micron felt filter on top of packed biomass
-
fill six 60 mL vials with fresh MCT oil
-
start pouring oil into top of percolator and start timer
-
Collect the bottom effluent back into the six 60mL vials
-
label the most potent vial “1,” the next vial “2”, the next “3,” and so on
-
stop timer once the 5th vial is filled
-
squeeze remaining oil into 6th vial
-
record masses of oil in each vial
-
mix and sample each of the 6 vials
-
empty percolator by removing bottom triclamp
-
reload percolator with filtration and biomass (batch 2)
-
sequentially pour in vials 1, then 2, and so on into the percolator
15.(a) wait for the liquid level to drop below the felt to pour the next vial
-
collect the effluent with stepped-infusion back into vials 1 through 6.
-
repeat steps 9 through 16 to collect data and run batch 3
Results -
-
The flower tested at 16.5% total potential (t.p.) cbd. I don’t know the cbd/cbda breakdown for sure because I think the Soxhlet causes a bit of decarb. From this potency, each of three 21 gram batches has 3465 mg t.p. cbd.
-
My first batch of biomass came back 57.84 grams and the second was 57.91 grams. The third batch of biomass came back 48.46 grams because I let it sit overnight while draining in to vial 6.
-
I only tested the first spent biomass potency at 8.68 mg t.p. cbd per gram, which, with 57.84 grams of spent material it means there remained 502 mg t.p. cbd in this batch. Thus from the input and output biomass potencies I infused (3465 - 502)/3465 x 100% = 86% of the available cbd. Not amazing but pretty good in my opinion… could do better perhaps with a finer biomass grind or using a little heat.
-
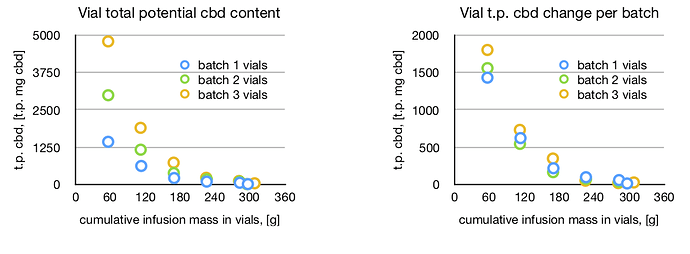
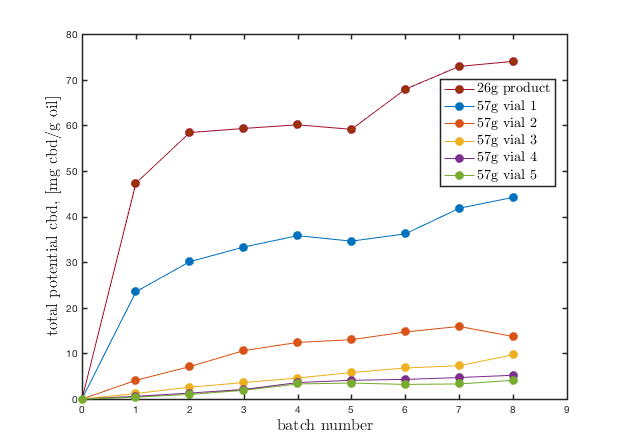
On the infusion side of things, vials 1 through 6 contained, respectively (61.4, 24.3, 9.4, 2.9, 1.5, 0.5) % of the infused cbd. Their potencies were (84.8, 34.1, 12.9, 4.0, 2.1, and 1.6) mg t.p. cbd/g. Notably you see that the vast majority-95%-of the infused cbd ends up in the first 180 mL of collected infusion.
-
When I look at the total potential cbd in all of the vials, at the end of the three batches, I count only 7800 mg t.p. cbd which together with the 3465x3 = 10396 mg t.p. input suggests an extraction efficiency of 75%. This efficiency is lower than the 85% I cited above but I also removed about 0.5 grams from each vial after each batch.
-
Looking at the potencies of the vials as a function of the cumulative mass taken from the system shows that, in my opinion, I could have done this infusion with 3 vials.
-
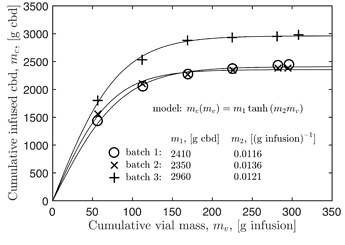
Really interesting to me is that the potency change after each batch is nearly the same for each vial. Looking closer, it seems to me that the most potent vials gained just a bit more cbd from batch 2 and even more from batch 3. To me this is a result of an increase of the infusion viscosity — or adherence to the biomass — because the flow rates of the more potent infusions slowed considerably for batches 2 and 3. So the simple gravity flow through the column gives some slight nonlinearity but it is mostly linear… from this data I can predict how vial potencies will increase for future tests.
-
The useful implication of this linearity is that one can design a process that runs well more than 3 batches to efficiently produce oil at a given potency. That is, if your spec is 30 mg/g then you start taking oil out of the process, i.e. vial 1, once it exceeds the spec. (In my experiment you could take out vial 1 after every other batch since you know it gains ~25 mg/g in potency per batch. Then, vials 2 through 6 become the new vials 1 through 5 and you fill the last vial with fresh oil.)
Happy New Year!
figs:
percolator:
start biomass:
ground biomass:
mid percolation:
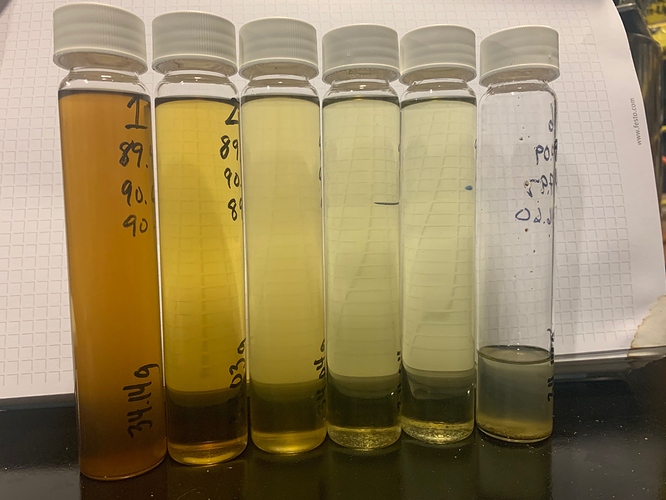
vials after second batch:
2020-12-30 percolation tests.xlsx (13.9 KB)
2020-12-30 percolation tests.pdf (40.0 KB)