Hey All,
I spend a decent amount of time lurking around the forum and I contribute where I can.
I commented on a post and got my ass handed to me from a chemistry perspective, so I figured I’d better dive deeper into the subject.
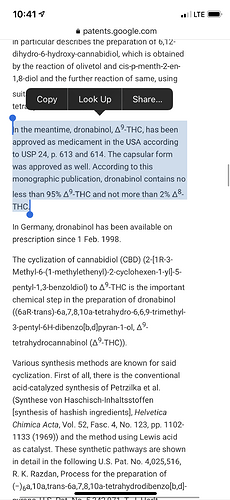
I see all kinds of people attempting to convert CBD to THC with very, VERY strong acids—often with little regard for relative weights of cannabinoid/solvent/reagent and often disregarding inert atmospheres or residual moisture in solvent. For these reasons, and because I happened to be down a rabbit hole on the topic, I figured I’d link the patent for the synthesis of Dronabinol, which is a D9/D8 compound that is quite pure. Typically 97%+ D9 with residual D8.
The really interesting thing about Dronabionol, is that the people who have created this drug found a way to convert CBD to THC with extremely high selectivity and great yields—and it seems extremely simple, relative to how it’s been presented in the forum.
I’ll summarize the steps below, but it is REALLY worth the time to read if you’re interested in pursuing this process. I haven’t done it myself, but now I’ve got to try.
-
Mix anhydrous solvent with CBD at a 1:1 ratio. There are options for solvent choice and desiccant that can be used to make the solvent anhydrous. Typically something like pentane, hexane, or heptane dried with sodium sulfate.
-
Optional—In a ratio of 3:1 Acid:Solvent or Acid:CBD, add Lewis Acid. This isn’t necessary but serves to improve yield and selectivity.
-
Inert the reflux atmosphere. This is critical. The author of the patent argues that residual H2O will reduce the selectivity to D9 and result in higher percentages of D8 in the final product.
-
Reflux with 4A zeolite molecular sieve. This blew my mind. The author argues that the molecular sieve is what is really allowing the cyclization to occur in the first place. The Lewis acid just potentiates that effect.
That’s the basics. Additionally, the manufacturers found that mixing the formulation with sesame oil was enough to prevent oxidation at room temperature for at least 3 months.
Anyways, I figured it was worth a share. I thought it was cool as shit and I don’t remember seeing this in other conversion threads. If this is just a repeat, someone toss it in the echo chamber ![]()