Be careful @Ozone … Especially if the product of that jar reaction is oddly colored (like neon orange)… Of course, since you dissolved it in NPS, I would guess you treated it with some heat and vacuum after formation, right? If so, it should be okay. If not, just beware that 9-chloro-HHC may be formed, which has not been tested in humans, afaik. ![]()
@TheGratefulPhil What is Δ4,8-iso-THC?! Is that monoterpene numbering or something?
Please do tell!
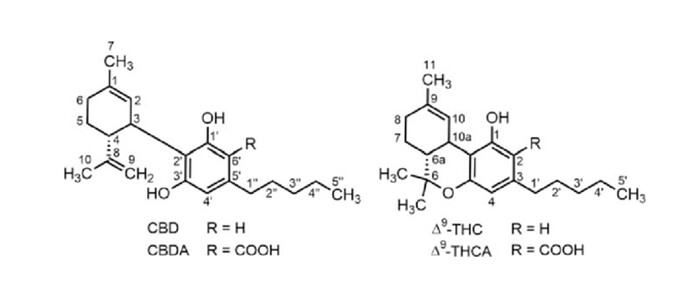
This is dibenzopyran numbering of CBD & THC…
Edit: Do you mean 4,8-iso-CBD?
I don’t think that is true. In CBD, the apparent ring strain of d9-THC that is relaxed upon isomerizing to d8-THC, that ring strain is not present.
I’m pretty sure that was shown early on. D8 is formed from D9 which is formed from CBD. That is the sequence of events.
The term intermediate has several meanings. Typically it refers to a chemical structure that occurs along a synthetic transformation, typically it is not isolated, although it often could be.
An intermediate can also be a stable, isolated chemical structure somewhere along a multistep synthetic pathway.
Not to be confused with transition state.
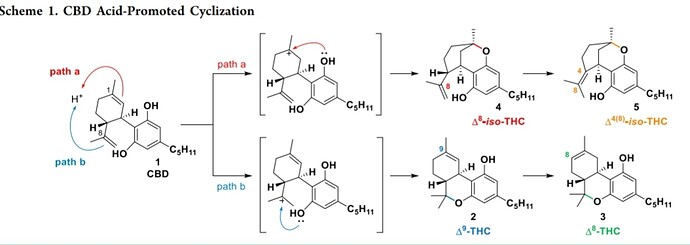
It’s the structure in which the wrong double bond took part in cyclization with one of the resorcinol hydroxyls AND also, that the isopropenyl double bond has isomerized and is now between carbons 4 and 8 in CBD numbering.
I laughed at that! Thanks for saying!
Anyway I learned a lot by reading this thread. Thank you so much guys. @TheGratefulPhil @mitokid , and everyone else
I did, too, as in hindsight is 20/20. I was way too ornery and what started out as an innocent gripe over choice of phrasing got a little out of hand. I pledge to be better.
But I am sure you and others are all too familiar with the getting out of hand tendencies on some of the juicier threads on the Forum.
It ain’t easy discussing/dissecting organic chemistry using an alphabet. What normally takes a whiteboard becomes really hard, particularly when individual posts seem out-of-synch, and especially when there’s no commonly agreed upon lingo/phrasing, etc.
I have no idea what you mean but I feel you
@mitokid I agree that strain is generally considered the driving force to D8 from D9, but we also have to take the locked hydroxyl group into consideration if the ring is closed first. I suspect that hydrogen can also play a drawing role in many cases. After all, when that ring is only single bonds and there is a leaving group (e.g. halogen) on the tertiary carbon (C9), the Δ9,10 pi bond is actually more likely to form than the Δ8,9 double bond. It is for this reason that I hesitate to call the strain “significant”. The substituted HHC molecule can, in fact, “relax” into the Δ9-THC configuration at temperatures from neg-60°C to +30°C.
I believe it is at least partially this behavior which causes equilibria between D8 & D9 products to arise under many CBD isomerization rxn conditions.
Do you happen to know the activation energies for the ring closure and the 9,10 —> 8,9 pi bond shift?
Do you know the difference in enthalpy between D9 & D8-THC?
I am asking because I do not have these values on hand.
![]()
That’s actually pretty cool I never realized that bottom double bond can isomerize and that’s how the other one is formed good looking on that little clue @mitokid
Edit: I think the top structure is named wrong, that bottom ring shouldn’t be closed
Next up Raney nickel makes iso s
The two pictures are the same, the bottom one is just upside down.
I just read a detailed mechanistic explanation of how the dehydrohalogenation of the the 9-Cl epimers occurs.
The top hydroxyl is then definitely close to the terpenoid ring, and if the 9-Cl is pointing “up”/in “front” of the paper in the way cannabinoids are typically drawn, or 9β-Cl, then it’s actually the deprotonated hydroxyl that abstracts the 10α proton and d9 is formed.
Yes, to the last part. It is reported as being 2.4 kcal/mol, corresponding to 97:3 equilibrium. Assuming that the transition state is symmetrical, ie., same in both directions, I expect the same difference in activation energies.
And surprisingly, the same values are found without the resorcinol hydroxyl. The paper is:
I just want to point out, this has a lot to do with the nature of the base and how it allows/disallows certain things to happen—especially as it pertains to the involvement of the “top” hydroxyl group on the cannabinoid molecule.
I think this is the info we all want but I haven’t been able to find it anywhere.
And I was going to edit my post and saying that I knew of at least one lab in the mid-West that are diligently working on the problem and may be able to extract data from Arrhenius Plots and get at the activation energy for the ring closure, or at least the rate determining step.
Whatever value is found, I bet it’s quite dependent on solvent since so far, everything points to disassociation of a CBD•catalyst pre transition state complex as being rate limiting.
The enthalpy difference is accurately reported in Razdan’s Hashish 26 paper and is a direct consequence of the d8/d9 ratio at equilibrium. I think that the difference in activation energies of both directions of this equilibrium cannot be much different from the ground state difference.
I think it has more to do with stereochemical requirements.
You may find most of what we are talking about in:
Special thanks to the @KingOfTheKush420 for drawing this article to my attention.
Thank you for the data and literature, @mitokid and @Kingofthekush420 ! It appears these documents have greater specificity and detail than the others I have read on these subjects!
@TheGratefulPhil , I’m unsure if you caught it, but that paper mentions what mitokid summarized: [The difference in enthalpy between D9 & D8-THC, I assume] is reported as being 2.4 kcal/mol, corresponding to 97:3 equilibrium. Assuming that the transition state is symmetrical, ie., same in both directions, I expect the same difference in activation energies.
So it’s about 10041.6 Joules, or 10 kJ… not too great a difference, I suppose, but seems bigger than I expected.![]()
Yes, @mitokid , that is what I meant. I probably wrote it incorrectly, attempting to keep it brief. ![]()
In the accounts of cannabis having been known by mankind for thousands of years, from physical proof, it has always been due to the detection of CBN. Even the stuff found inside the Pyramids of Giza was basically all CBN.
What ultraviolet radiation does is that it increases concentration of singlet oxygen, but it’s not absolutely necessary. Time and air will turn d9 to CBN.