I was just reading about the Baeyer–Villiger and Dess-Martin oxidations. Are these basically what the chloranil achieves?
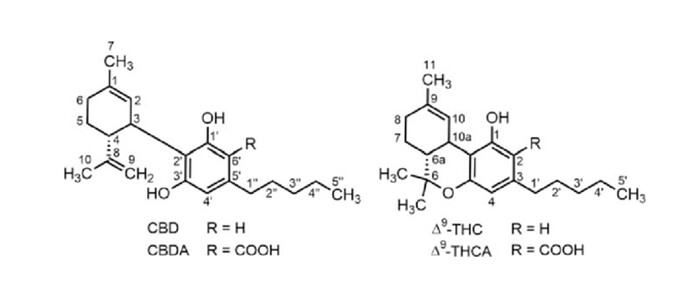
Which of Adams’ papers? This might be before they nailed down the position of the double bond in CBD and the chemical structures might be incorrect/guessed. That would clearly be indicated in the text of the paper.
No. Quinone mechanistics are weird. Well maybe Dess-Martin has some similarities in terms of electron transfer, not sure.
Quinones are like acceptors of two hydrogen radicals in a one-at-a-time kinda way. Oxidation of d9 definitely involves radical intermediates. That has been proven.
There’s a patent
If you knew what they replaced it with you’d laugh
Thx @mitokid
Oh…do tell
![]()
Dietary supplement FTW ![]()
![]()
![]()
I have a feeling this is how most are doing it
Whos gonna try koh with 1% dmso and heptane?
Hydroxides are strong enough to make d10
I tried koh in methanol and got 2% d10 but I didn’t have dmso in there to break apart the base (no idea if this will work but if dmso can grab a potassium ion from potassium t butoxide maybe it can do the same with koh)
I’ll try it
Make sure to use d9, d8 can’t do the same mechanism from my understanding
I’ve only got 8 handy. I’ll try it anyway and document via hplc
Use the highest d9 disty you have
Atleast then you should see some of it convert
If if does
It won’t work. Without the double bond between 9 and 10, the 10a proton ain’t sufficiently acidic to be abstracted by an alkoxide.
My lab is in a non legal state for working with D9. We are legal for possession, but retail won’t come until late next year so I’m stuck with working D8 at the moment.
Suppose someone else can try it though?
I’ll order dmso
DMSO is used but doesn t work
Albright! Check this out!
250 mg of CBD, 2 ML distilled H20 and 3 ML 30% HCL.
Put it all in a glass jar with a air tight lid.
Heat the jar at around 60 C for 8 h (a cup-heater works well).
Add 100 ml of water and 10-15 g of bicarbonate to neutralize the acid.
Dissolve the oil in NPS
Wash the NPS with water 3 times.
Separate NPS from water
Separate NPS from THC…
Feels pretty euphoric buzz. I haven’t smoked pure D-9 but this batch feels more euphoric compared to a glacial acetic acid and h2so4 batch.
![]()
@pdxcanna @mitokid Please excuse my old and literal thinking. I meant that although D9 is a potential precursor of D8, it is not an intermediate by the chemical definition which I had learned. I had learned that true intermediates are not stable; things like carbocations, for example. However, it seems that the definition has relaxed since I learned it to include stable products within a stepwise reaction. In that sense, D9 could be considered an intermediate in many CBD —> D8-THC reactions.
One point to the contrary, however, is that the energy required for the heterocyclic ring closure and the energy for shifting the position of the double bond are apparently VERY similar. Therefore, the intermediate (in the new sense) could be “D8-CBD” (actually numbered differently, so “D8” is Δ1,6-CBD & “D9” is Δ1,2-CBD) OR D9-THC… or both reactions could occur simultaneously!
Good catch! I learned a new definition, so thank you both!