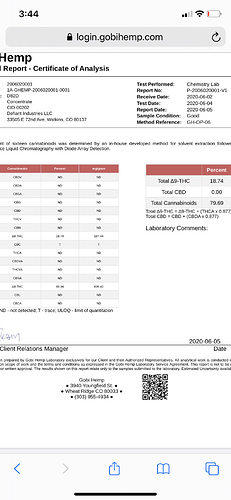
All solutions I can think of is or more acid use or longer reflux time to lower the D9 numbers
Temp is going to be the boiling point of water at your altitude.sorry missed that question.
I’ll try both of those. I’ve switch acids to Tosic but I’d still like to figure out citric in my free time. Still bugs me that we never figured out the potency issues we were seeing when we were winterizing in Acetone.
I’m going to have to try that very soon. Great contribution Jed!
Edit: What solvent did you use for your dry reaction? I’ve never used citric acid to yield d8 but when using zinc chloride to yield d9/d8 I haven’t seen anything I would describe as violent. I use n-hexane and more recently n-heptane.
Just isolate and citric acid. 8% by weight refluxed at 180c lots of side reactions at that temp though.
Same 95% isolate.
Most of the volatility could be contributed to the fact that my mantle is junk and won’t spin a big stir bar to break up the bubbles.
Any loss in mass during either the dry run or the wet run?
I haven’t been calculating losses yet but neither seemed to have any real losses other than what’s left in the short path which seems to be maybe 10 grams from a kilo run. That’s usually only left because it’s the end of the day and need cool down time.
Mol sieve in dry reactions buffers out side reactions in dry setting. Similar to what the water did.
Sometimes it’s better to be lucky than it is to be good
@JedClampet just poking fun, I really appreciate the data
It is possible that the ph of the water is changing your results. As far as d8/d9.
interesting question. @JedClampet did you use purified ph adjusted water or tap water? Nice work!
Lol that’s true. I’ve been pretty lucky my whole life. It could also be that I listen when people tell me things and I’ve got a nack for applied theory lol.
Distilled water. I suspended the citric acid into the distilled water then added to isolate that was warm enough to be a liquid.
That would be my assumption. I didn’t expect it to work because I read somewhere on here that the solvent used for a reaction like this needed to have a pka of 16 or better where as water being my solvent is in the 15.3 range I believe. I believe PKA was the right term.
I guess what puzzles me Is why it’s so hard to control the double bond on the top ring. Obviously the citric acid will pull that hydrogen at the bottom and close the ring but what’s up with the double bond at the top… my theory was that I was putting to much energy in to the reaction causing that double bond to become unstable and therefore bounce around.Thats what led me to water with a lower energy input. Once again I have no chemistry education so I could be way of the mark.
depending on weather the the compound that is created is destructive.
an idea to start with anyway
I think you may find a precipitation test in a two stage attack on the CBD
to a quinone then a hydrazone.
the hydrazone would possibly be soluble in water.
the oxime may also show to be insoluble in some solvents that THC will disolve in.
I doubt a bisulfite complex would work with the experience I have had with
the complex.
It normaly wants a rather pure compound to form and precipitate.
Polarography might help as well as one could run the sample pre oxidation to
the quinone then post oxidation and run a formular on the two results to calculate
the difference.
Polarography is used to measure some ketones in perfume though
I can see it also acting on the double bond in both THC and CBD but the difference
between an oxidised sample giving the quinone and the raw sample might
be enough to work out the CBD content.
[21910189 - Reviews in Analytical Chemistry] Determination of Phenones in a Perfume Composition.pdf (595.3 KB)
ill keep thinking on this one as the name reaction and wet chem is a passion of mine.
I am still far from a master though ![]()
till then I think that we can probably develop at least some kind of preliminary testing
with optical and beams (which goes to quinone :)) that uses cheap as parts
but ye I thought that was the case and only chromatography was the go at the moment.
I also think raman could be tweaked to do this too and I think a diy raman would not cost much
but the code behind the fourier and logic behind the quantification would be quite
complex.
thankyou for answering it means a lot to me.
Very cool results, I would have never thought of using water.
to keep everything accessible in one place
AgTonik’s Cannabinoid Isomerization References
You are the man and this is an awesome subject. I wish I could Google fast enough to keep up with the chemistry but some sort of even vaguely quantitative reactive precipitation assay would be a game changer.
another idea for testing would be the use of nafion polymer to isomerise to Delta 9 in solvent.
the nafion would be able to be reused and easily extractable leaving no acid to be
separated from the cannabinoid solvent mix.