I’m gonna take a wild guess here about the EtOH vs CO2 extraction capability. Perhaps frigid ethanol isn’t so great at grabbing CBDA? As in, maybe the amount of money required to get a kilo of CBDA reflects less on CO2 than -70 ethanol.
I’m wondering if you could explain this a little more.
How is extracting with CO2, then winterizing (with cold ethanol) a better route than cryo-ethanol?
You mention you took your ethanol extracts to distilate. was that because you were extracting at rm temp and getting black goo that needed cleanup before presenting to the customer?
Little late to the party but I am seeking answers and would love some feedback. This is what I believe to be CBDA crashing from a hydrocarbon extracted crude. It was several 5lb runs of fresh frozen cherry mom mixed with dried frozen trim machine dust, cold solvent without dewax, recovered to slight vacuum and pushed out the bottom drain with nitro. We have sent away samples but we’re having some strage issues with labs from it. I believe we will have a solid test result on this oil soon. I did not try to force crystallization, in fact the oil was vacuum purged after an overnight preheat to 95°F for almost a week.
My questions are: Can I wash these crystals the same as THCA with cold solvent on a buchner? If so, which solvent would allow me to recover the terp portion as well? And lastly is isolating CBDA worth the effort?
can confirm. have inadvertently crystallized CBDA, purified those crystals further via crushing/washing and got a white solid with a distinct melting point.
Sorry for the delayed response, I’ve been away from this forum for a while.
I wouldn’t necessarily say it’s a “better” route, but for the product we were trying to create it made the most sense to go the CO2 route for flavor and yield. It’s a strain specific, flower sourced, oil cartridge based on the Canna-Tsu CBD strain.
And no, our ethanol extracts are some of the lightest you will see on the market (comparable to distillate at times), but crude ethanol is ready to go straight into a SPD boiling pot, whereas CO2 takes more post-processing before SPD.
I don’t think you can get distillate without SOME decarboxylation.
![]()
Did they crystallize?
Any tips on ratios for CBD distillate carts to keep from crystallizing?
Did you get a reply to your question here? Nice looking resin ![]()
What temp was the melting point ? Just curious cbd is like 62C
yaknow I cant remember offhand, its been some time - Ive got it written somewhere Ill see if I can find it… My highest CBD melting point was something like 67 C via painstaking recystallization to attain some insanely high potency seed crystals, but I very much trust my result. A typical CBD first crop crystallization however I see 62-65C, so I think you’re probably making pretty decent product by that measure.
FYI, CBDA melting point was as low as 81 C before wash/recrystallization/drying and as high as 85 C after washing/drying
What exactly would you like me to confirm my friend?
The melting point of cbd-a sir ![]()
I do not think it is a crystal at room temperature and pressure.
Hmmm interesting cbd-a not a solid at room temp ?
I dare not doubt your opinion but then what is it that people see cristelizing then in un decarbed extracts ?
Maby deu to accitic conditions it is possible
I personally have never seen any but seems several have
You may have a mixing problem and not a ratio problem. If you dont mix evenly/enough you will have some spots within your mixture that still are high in cbd content.
cbda is sappy. I have extracted high cbd strains that are very sappy in texture
I have never personally seen a cbd-a isolate that is convincing with evidence to support its chemical structure. I would require IR and/or NMR personally to confirm the presence of the acid.
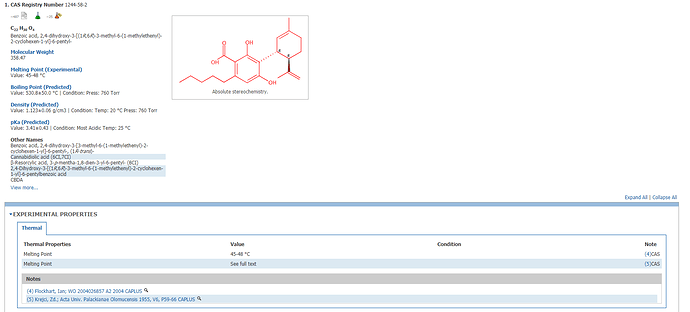
This is what the scifinder search yielded for me.
Even with pretty stellar academic credentials (my university allows me to access the vast majority of chemical literature I cannot access these on my first try)