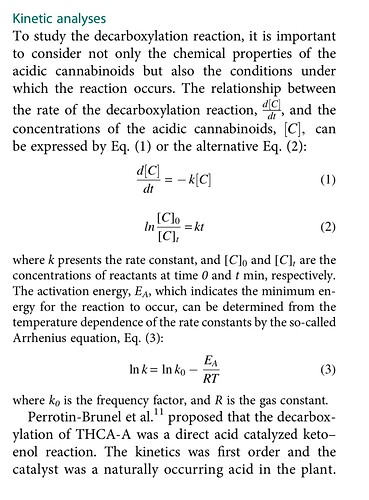
Introduction:
Obviously, there are a number of ways to decarboxylate BHO/Rosin while preserving the natural terpene profile; this is just a simple method. There are numerous ways to expand upon this idea, which is why it has been published in this open forum. This SOP, if followed correctly, will produce +12kg of fully decarboxylated BHO in about an 8 hour shift, more or less. I indicated a 6" diameter vessel specifically for its’ higher pressure rating which should allow for safer operation. That being said, this SOP is entirely theoretical and has never been tested, to my knowledge.
A few science points:
-
The vessels and spools are sized specifically so that they will not be more than 20% filled with BHO. This is by design; so that the pressure in the vessel should not reach critical levels at the designated temperatures.
-
Always pull a complete vacuum, as deep as you can get, on the vessel before decarboxylating. This will speed up the entire process and remove most of the oxygen from the vessel to prevent any unwanted/uncontrolled organic reactions/oxydation.
-
Tuf-Steel gaskets are expensive but are probably the best available material for this purpose. They have serious heat and chemical resistance, which is required for this process. They should also be torqued appropriately with a Torque Wrench (50in./lbs).
Abstract:
Cannabinoids, in their acidic form, will crystalize over time under normal atmospheric conditions and prevent a homogeneous solution from occuring. Also, the primary byproduct of acidic cannabinoid decarboxylation is CO2 gas, which distinctly affects the olfactor sensory organs; specifically, it has a numbing effect that deprives the user of a complete entourage experience and requires a higher temperature for vaporization. The CO2 gas may also deprive the user of oxygen during consumption, thereby affecting the overall experience.
The final process of degassing the oil will require much less energy as the butane/isobutane/propane-THC bond is much weaker than with THCA. As the BHO is now a mostly-homogeneous liquid, it may be easily degassed by any variety of thin film evaporation techniques. Further homogenization could require equipment not listed here.
Once the oil has been fully decarboxylated, degassed and homogenized, it wil vaporize at a consistent lower temperature and provide the consumer with a full entourage effect, unique to the extracted compounds. The subtleties of the terpene profile will be expressed with a degree of purity as true to the plant as possible, at low temps. Recommend 435-485F.
Considerations:
-
Use teflon tape on your threaded fittings. If that isn’t enough of an explantion, please do not attempt this process!!!
-
BE SAFE!!! I cannot stress this enough, heating volatile organic compounds ina pressurized vessel should only be performed under controlled conditions only by qualified individuals. If you question your qualifications, please do not attempt this process!!!
Safety Equipment:
- Eye Protection
- Chemical Resistant Gloves
- Chemical/Splash Resistant Lab Coat
Equipment List:
-x1 C1D1 or C1D2 working environment
- Booth, Hood or Explosion Proof Room
-x1 2-Stage Rotary Vacuum Pump
- with fitting and hose
-x1 Recirculating Heater/Chiller Bath
- Thermo Fisher Arctic A25 Refrigerated Circulator (AC150-A25)
-25C - +150C, 12L, 115V/60Hz - with appropriate thermal fluid in appropriate volume (+12L)
-x2 4-way Manifold (High temp threshold +100C)
- with fittings and tubing for Recirculator Connection
-x4 6"x8" Stainless Steel Tri-Clamp Jacketed Base
- with appropriate fittings and tubing for Manifold connection
- I would recommend quick-connect fittings rated for appropriate temperatures
-x4 6"x32" Stainless Steel Tri-Clamp Spool
-X8 6" Tuf-Steel Gasket
-x4 6" Tri-Clamp Vessel Cap w/ fittings
- Collection Pot or Diamond Miner Lid
-Fittings:
-Port with Ball-Valve
-with fitting for vacuum hose
-Pressure Relief Valve (200psi)
-Compound Gauge (-30"Hg - 250psi)
-Max temp +110C!!! (very important)
-x8 6" Tri-Clamp High Pressure Clamp
Preparation:
-
Connect the Recirculator inlet and outlet each to a 4-way manifold.
-
Plumb each 4-way Manifold into the Explosion Proof Environment.
-
Ensure adequate clearance in the Explosion Proof Environment for the height of the decarb unit (approximately 48").
-
Connect each Jacketed Base to each inlet and outlet manifold.
-
Fill the Recirculator resevoir with 12L thermal fluid.
a. Begin circulating fluid.
b. Top off resevior as fluid fills Jacketed Bases.
c. Stop circulating fluid, disconnect bases.
Process:
- Fill each Jacketed Base with 3-3.5kg degassed BHO.
a. Ideally, 1500ppm or less residual solvent content. - Assemble each unit by attaching:
a. 6"x32" Spool with Tuf-Steel gasket and High-Pressure clamp.
b. 6" Vessel Cap with Tuf-Steel gasket and High-Pressure clamp.
c. Using a Torque Wrench, secure each clamp with 50in./lbs torque. - Place each unit in the Explosion Proof Environment.
a. Connect each Jacketed Base to the appropriate inlet and outlet hose from the manifold.
b. Set the Recirculator temp to 15C.
c. Start circulating thermal fluid. - Connect the vacuum pump hose to the Decarb Vessel.
a. Turn on the Vacuum Pump.
b. Open the ball-valve.
c. Once maximum vacuum depth is achieved, close the ball-valve and turn off the pump.
d. Disconnect vacuum pump hose. - Set Recirculator temperature to 110C.
a. Monitor the pressure in each vessel as the temperature ramps up, if the pressure begins to approach 200psi:
-
Adjust the Recirculator temperature to 15C and allow the pressure to drop.
- Once the temperature has arrived at 15C, allow to circulate for 30 minutes.
- Open the ball-valve to vent excess CO2 gas to 0psi, close the valve.
b. Once the Recirculator arrives at the set temperature:
- Allow 110-150 minutes for the decarboxylation process.
- The vessel pressure should not exceed 150psi.
- After 110-150 minutes at 110C, set the Recirculator temperature to 15C and allow to cool.
a. The material should be completely decarboxylated.
b. Once cool, open each ball-valve to vent any remaining CO2 gas.
c. Close each ball-valve. - Attach the Vacuum Pump Hose to each miner, respectively.
a. Pull a full vacuum on each Decarb Vessel.
b. Allow to sit for 2 hours under full vacuum. - After 2 hours under vacuum, open the ball-valves on each miner.
a. Set the Recirculator temperature to 35C, start circulating thermal fluid.
b. Disassemble each vessel.
c. Once the decarbed oil is at temp (35C):
- Disconnect vessel from manifold
- Open ball-valve and remove cap.
- Transfer to Ball/Mason/Kerr jar for storage.
Summary:
The primary byproduct of decarboxylation is CO2 gas, which is problematic when attempting to decarboxylate BHO in a closed vessel. By placing the material indended for decarboxylation in a pressure rated vessel and creating a vacuum environment, the material may be fully decarboxylated while preserving the natural terpene profile due to the available headspace and oxygen-deprived environment. The additional pressure created by the CO2 gas will allow any volitalized terpenoid compunds to reflux under pressure while cooling, thus allowing the preservation of the unique terpene profile. Once processed, vacuum boiling of dissolved CO2 gas in the oil should aid in the degassing of any residual hydrocarbon solvent.
The Recirculator should cost about $6k, brand new, and each decarb vessel shouldn’t run over $1.5k. All told, so long as you have a C1D1 or C1D2 environment available, this setup should cost about $12-$15k to yield +12kg ready to consume product per 8-hour shift. This is potentially the highest margin product in your inventory, right now.