If I understand this correctly AA can hold more water, can be recharged with heart the same as 3a and it’s a fraction of the cost. Am I missing something like 3a picks up additional undesirables in this application which makes it a better choice?
What is this AA you speak of?
I’m curious as well, heard of 3a, 4a and 5a but never 2 ![]()
Pretty sure he’s talking about activated alumina!
" WHAT IS THE DIFFERENCE BETWEEN ACTIVATED ALUMINA AND MOLECULAR SIEVE?
“While they often perform the same process of adsorbing moisture, activated alumina and molecular sieve differ in many ways. Molecular Sieve features pores whose size can be adjusted by ion exchange, allowing them to separate molecules based on size discrimination, a task other adsorbents are not capable of. This is why, for example, molecular sieve is used in the separation of ethanol and water. The sizes of the pores of activated alumina cannot be tailored and are not necessarily uniform. However, activated alumina has its own pros. Activated alumina shows a higher affinity for water than molecular sieve, so it is often used in the drying of various gas and liquid streams, instances when it is important to adsorb the most moisture possible. Its high crush strength allows activated alumina to withstand the high pressures of industrial applications.”
Don’t mind me, my mind went to 3a sanitary requirements.
Sorry @terplord420 got it right, ACTIVATED ALUMINA. Im no guru by far but from my reading I thought that abbreviation was standard jargon.
Thanks for the info. Based on that info and our application of removing specifically water I don’t see how adjusted by ion exchange is benificial if all we are after is the water and activated alumina with it’s non adjustable ion exchange will still picks up more water.
13x is great for stripping terps as well as moisture from your solvent, GMO always leaves my tane smelling like hot ass stew if I don’t CRC or 13x sieve.
To piggyback on this with a related topic, if one uses nitro assist and is passively recovering from collection tank, through a molsieve and into the solvent tank after burping excess nitro pressure out of collection tank (never to 0psi), there would inevitably be remaining nitro flowing through the sieve along with the gaseous butane correct?
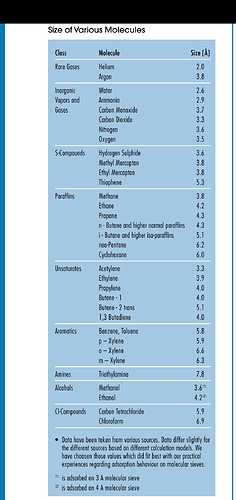
Assuming this is true, nitrogen has a molecular diameter of approx 3.7 angstroms so wouldn’t the 4A, 5A, and 13x sieve beads adsorb the nitrogen as well as the water and other unwanteds? My concern here, if my logic is accurate, is that the nitrogen would fill the pores in the sieve beads and block the adsorption of more water.
Water molecules are approx 2.75 angstroms so the 3A beads would work for water while also not adsorbing the nitrogen. I am probably thinking about this incorrectly since I have read many comments by vastly more experienced extractors than I saying they use a mixture of 3A, 4A, 5A and 13x but I would still like to wrap my head around the explanation here. Thanks!
I’ve never tried myself but have been told by pros here nitro will recover. And they use a sieve for every run. It will be slow but my understanding is some will go in
Are u looking up liquid nitrogen or nitrogen vapors. Bc w nitro bump you only get nitrogen vapor tank not LN2 . there is no liquid in the tank
Yes nitrogen vapor. The N2 molecule and the H20 molecule are both small enough to be adsorbed into a 4A and larger sieve bead that’s why I am wondering if that renders the 4, 5 and 13x beads less effective than the 3A at adsorbing moisture if they’re already “full” of N2. Or is there not nearly enough N2 to “fill” the beads?
But like you said, I also have seen that the pros use a mixture of the different beads but it just bothers me when I can’t figure out the “why”.
I would introduce the solvent that needs to be dried in the order of smallest angstrom to biggest.
Id imagine the 3a will catch as much as it can and w/e doesnt get caught in there just keeps retrying until it gets to the 13x. If you go biggest to smallest most of ur 13x would get filled up with stuff that should be caught in the smaller ones, then things that should have been caught by the 13x are now passing through, because the 13x is saturated.
Now that i do more research, i see what your saying. Most info states that 13x is best used to purify oxygen from the air by catching the nitrogen in the beads.
Looks like we should probably be pushing our solvents around with helium instead? ![]()
Please no!
Until we learn how to make more, gratuitous use of Helium should be kept to an absolute min.
…and we need to learn how to make more soon, or we’re never getting off this rock.
Why not use 13x for your mol sieve?
3A just grabs water. Anything larger also binds to hydrocarbons. They can be used between oven and vac pump to keep that exhaust clean, but if you put 4A and up in your extractor it’s will fill up quickly with your solvent
I’ve got some 13x and what I’ve found is that it can adsorb quite a lot of solvent… it should not be used in as great of a quantity as 3a mol sieve is due to the that fact.
I don’t run solvent across it except when I first load it to clean it. It’s meant for vapor in this instance
Right, I use it for vapor… that’s when I saw it picking up solvent
Interesting? I’m not sure if I’ve noticed solvent loss. I’m going to pay closer attention now.
I wonder if stepping down to 10a would resolve that