Hey guys!..Me again lol.
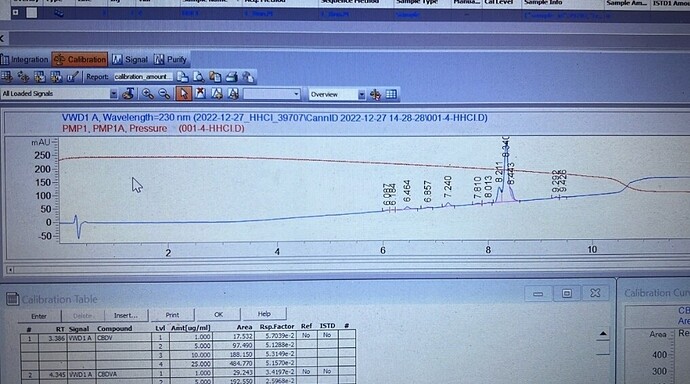
Has anyone ever seen this before? I have been doing my best to improve my our HHC procedures. When I put it my jars in a cool dark location within 24 hours this stuff starts to crystallize. It freaked me out at first so I ran it through the HPLC and the peak is a little over 90% 9R-hhc there is a small S peak still. I attached some pictures. I just have never seen this before. Is it normal? Is there a market? What do I call it? HHC isolate? lol I saw a few in marketplace claiming HHC isolate but it was all oil. Never rock like waxy substance.
throw that hhc in 5:1 methanol or ethanol solution for me if you do not mind.
Unless you already have.
I would try to crash it out in some pentane and see if you could make isolate
I crashed it out in ethanol that’s how I got that rocky substance you see in the picture
Congrats.
Is that what you tested, the rocky substance looks like fats and waxes could be wrong just by eye check.
Finally some real science happening here!
No just the oil. It’s hard for me to test the isolate but I sent it off. It almost destroyed my HPLC column. Ok heard but that should be impossible. Its CBD isolate converted into D9 Converted into HHC. How on earth would that be present? Raw source material was 99.3% CBD.
Remember in Germany we can’t deal with cannabis like you guys can.
Just a theory…
You may have stumbled on a polymorph crystallization. Maybe there is a ratio between the mirror image molecules that create a binding affect.
https://pubs.acs.org/doi/10.1021/cg300398a
Didn’t read this but just a guess
Also maybe worth noting that even when chromatographed to elute anything over 20% 9-S even if 98+% total HHC, the solution does not crystallize. Same source material. Slightly different process parameters. Namely the enriching of with varying amounts of THCA. Anything over 85% 9R however will crystallize in about 24 hours. Go home, go to bed, come back to work and voila crystals. No additional solvents nothing. Just Mason jars mostly blanketed with nitrogen.
I wonder if a metamorphic field will replicate this in other labs.
Totally a ratio here for crystallization. Super interesting. Finally a new mystery.
D10 will crystallize, I wonder if maybe something to do with the enatiomers themselves is causing it
Maybe 9R will crystallize and 9S won’t so when you get high enough 9R this happens
As far as I know both isomers of d10 crystallize
Agreed.
There is a synthesis for pure 9R from a specific chiral citral molecule, what would be fun is to synthesize it then see if this happens
Mitokid and me use to talk about this synthesis as a way to make pure 9R hhcp
RIP @mitokid miss you and our conversations man
You could crash em out and see.
I’m thinking it’s a ratio with a binding spot. Think a snowflake.
Maybe S is the center of the Micelle and the R is the outside binding?
Also he would know immediately.
Dude will always be the smartest chemist I ever encountered
His knowledge was out of this world
15,000 hours of lab time. Can’t beat that.
They say it takes 10000 hours to master something
He would have been a grand Master then xD