What is the actual chemistry reason for this? Like what is the chemistry involved that requires GC for the separation?
Pretty sure it has something to do with the difference in boiling points making them easier to seperate
@kcalabs might be able to chime in on why they use gcms over lcms for these kind of seperations
@Bret_HoneyGold A little confused on your end goal here.
Are you setting up ISO 17025 accredited in-house testing for MC Nutraceuticals?
Or is this just simple in-house analytics for QA/QC purposes?
@kcalabs would love to hear the actual chemistry reason why GC resolves cannabinoid isomers better than HPLC
I think it’s in-house QC for their company. It sounds like they want someone who will:
a) set up equipment like HPLCs
b) develop methods related to cannabinoid isomer resolution
c) manage employees
d) write SOPs
e) do formulations of products
So it sounds a little like a hybrid role. Part analytical chemist, part formulator
I thought so too until I saw the “ISO audit” part; while not a direct requirement, it’s still making me think that they have eyes on the former…
Which would have a BIG conflict of interest, at least from the perspective of the accreditors and auditors.
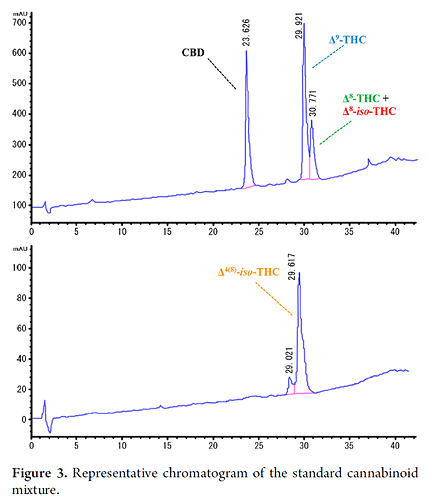
you have co-elution with cannabinoids in traditional analytical quantitation methods. for example, d8, d9 and exo (d9, 11) all will co-elute on the 75:25 ACN:water 1ml/min isocratic quantitation methods that are marketed with the c18 columns that companies like restek sell.
with a GCMS, you can actually look at the fragementation patterns of the cannabinoids. one peak from a compound on a GCMS gives you several different ‘fragments’ of the compound itself. when looking for isomers, d8 and d9 for example, you can just click on a peak and look for a primary fragment at 299 m/z (d9) vs 231 m/z (d8) and immediately know if that peak is d9 vs d8.
check out this paper that goes into more detail about how a gc ionizes cannabinoids and how their fragmentation patterns are unique relative to the ionization process in the first place. when I first started working with a GC, this was the paper that really opened my eyes as to how the technology works. It’s still unbelievable to me that people actually turned this thought experiment into a piece of equipment and I’m no expert by any means with a GCMS; there’s more to it than I don’t know than I know harvey1987.pdf (4.6 MB)
I get all that. I know how mass spec works, I used LCMS-TOF-ESI for analyzing oligonucleotides. My question is really concerning the separation aspect (in the case of GC the column and oven). The detector is kinda irrelevant for this my question.
What I would like to know is, what is it about the separation module on a GC that makes it more effective at separating those compounds as opposed to HPLC? Because you gotta separate your mixture before it’s sent to the detector.
You can have an MS as a detector on a HPLC just as you could with GC. So my question isn’t concerned so much about the detector, I am more interested in the separation aspect prior to analytes going to the detector.
I could be wrong, but I think boiling points of these isomers are could to be pretty similar, as the molecular weights are similar, so I don’t think that physical property is being leveraged for separation but I could be wrong.
Good point. My experience has been employers will typically ask for about everything under the sun because it’s “nice to have”, but on the job you’ll typically do only a fraction of what the wanted
the GC column KCA recommends (65%methyl / 35% phenyl polysiloxane) is different from the traditional c18 column used on an LC canna method.
I have never seen that type of stationary phase available for a LC column.
either way separation on GC in a combo of boiling point and polarity interactions between mobile and stationary phases. With LC, I like to think of solubility and polarity interactions
QA/QC , any many other responsibilities.
That makes complete sense. Thanks for the response!
The GCMS allows for more fragmentation for better identification compared to the LCMS.
RP-LC methods look at difference in lipophilicity. There are basically no differences in polarity between those isomers. GC uses temperature along with interaction with the chromatographic bonded phase for better separation.
Our team has been testing for the presence of novel drugs and drug metabolites for many years, so what we offer is beyond the capabilities of many labs, including in-house labs.
@IonStorm to add to @kcalabs and @ShuckleBerryFinn comments, I think this has also to do with the fact that one can use very long capillary columns for GC.
I use a 30 metter column.
I’m interested in seeing the 30 minute HPLC method’s chromatogram. The Marzullo et al. Natural Products paper from 2020 convinced us it wasn’t worth the development work:
Higher amount of theoretical plates in a GC column.
Much higher then in an HPLC column.
Main reason is that the column is much more narrow, resulting in less eddy diffusion.
The length also helps. An HPLC column is something like 20 cm max and you can get a GC column that is 60M. Additionally there is difference in film in a GC column vs packed particles in an HPLC column.
Anyway, all this results in a GC typically having a much higher resolution. So if you have compounds that are chemically very similar, GC can often separate stuff that an HPLC can’t.
Honestly I think LC can separate these isomers with some creative column selection. As in 2D-LC with c18 combined with an amylose column. Just nobody has paid me to do it yet…
Yah I am not convinced that it cannot be done on LC, especially with some of the interesting columns out there
Couldn’t smaller particle size increase theoretical plates in an LC column?
It does, but its still not even close to that of a GC column. You can def optimize a method on LC and might be able to do it. But yeah if you really want to get into all the chiral isomers you pretty much need chiral chromatography. Its very difficult to separate some of those unless either your stationary or mobile phase is chiral as well.