Just looking for a little bit of advice wrong those of you that have used nitrogen or argon gas flow in a system. I am running reactions that are not under vacuum and flowing in with nitrogen and then the nitrogen exits the system through a gas bubbler. I find that I am going through a 200 cubic foot tank and a day this way and I am flowing the gas at less than 1 psi, what am I doing wrong to where I am burning through my gas so fast and do I really need to have a continuous flow when there’s no way for air to get back into the system because of using a gas bubbler. Thank you any help would be appreciated
You need a flow meter. If you have driven out the air of the system the inert gas can be turned way down, or completely off.
Check out the schlenk line survival guide here:
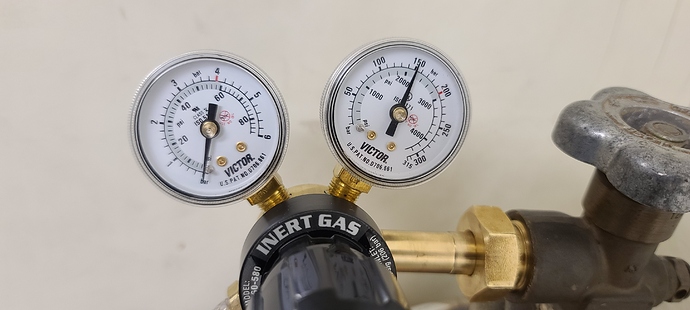
@Soxhlet beat me to it but it’s an important lesson for people using inert gas, especially with glassware. Ideally you would use a two stage regulator to be able to reliably set the pressure in the inches of water range so that you don’t pop joints. Regulators that output upwards of 100 psi are not suitable for something like a schlenk line because bumping the valve can be expensive and dangerous. Lastly, if you want to control gas flow, you also need a flow regulator. Not instead, in addition
Excellent thank you! I figured the basic regulator I was using wasn’t enough and also being able to just shut off the gas. Thx
I bought a regulator from The Keg Depot and now I will pick up the mechanical flow meter too attached to that?
I was also kind of wondering if a standard CO2 regulator from let’s say Titan controls would work
Most of the ones you will see with a flow meter don’t actually regulate output pressure, so if you have a perfectly sealed system you can quickly get 100 psi into it which will pop your glassware like a tick. You want to look for a regulator with an output range of 0-10 psi or so and then add a flow meter to it. I’m not sure if Titan makes one that low but there are quite a few options and the cost isn’t any different really
You can’t adjust psi with a titan, only flow. I wouldn’t consider a titan a standard CO2 regulator. They’re great for the grow room though.
Regular CO2 regulators can be used with a CO2 regulator to nitrogen tank adapter. Only makes sense if you have a CO2 regulator on hand though as there’s not much of a cost difference for CO2 vs nitrogen regulators. You will also still need to pair it with a flow meter. They make regulator/flow meter combos but the ones that actually regulate psi aren’t cheap.
This is the regulator I currently use and I will order the flowmeter requested above this should work correctly?
The delivery psi on that is pretty high. I would recommend finding a regulator with a 1-15 psi delivery pressure.
Still though where does the flow meter go?
Tank to regulator to flow meter
Is anything rated for nitrogen, also work with argon?
I think in 90% of the situations we probably wanna use argon instead of nitrogen
From what I know nitrogen is good for sparging and argon to blanket on top of a reaction, new to inert gas tho so I’m sure someone else will chime on that but also I was told my regulator will work with nitrogen and argon.
Usually they are interchangeable, they use the same CGA fitting
I think in both situations wether bubbling through the solvent or blanketing on top, i think argon should be more effective at pushing oxygen up and out. I cant think of a situation where oxygen needs to be pushed down and out.
Maybe when the vessel is empty, fill with nitrogen and open the outlet valve to push oxygen, down and out, then reverse argon into the bottom to push the rest up and out?
As far as the gasses settling or not, I really don’t think there’s to much of a difference. The reality is that it’s pretty easy to displace the majority of the air with whichever gas you use. The more important factor in my eyes is equilibration time to get the dissolved oxygen to exchange and be replaced with inert gas. By sparging it happens nearly instantly, but it’s not necessary if you’re patient before starting the reaction
you can use either with that flowmeter, the only difference are the calibration lines. The flow meter is calibrated to the density of the gas. You will only see minor differences.
ASME pop quiz: do you read from the bottom, top, or middle of the ball?
Answer: depends on the manufacturer
also bobbin vs ball are read differently