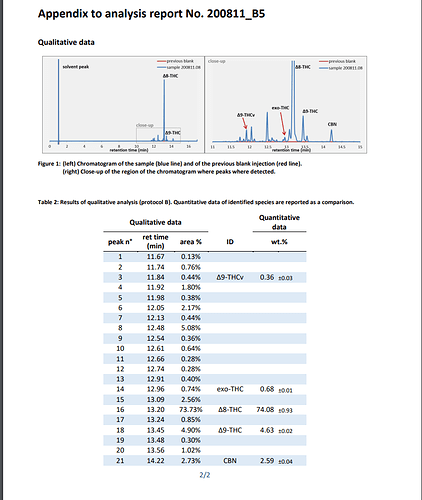
After CBD isolate conversion to D10, D9, D8, CBN, EXO, ect there is still a significant amount of matter that is not part of the cannabinoid potency.
Ex. I performed a D8 conversion from 99.9% CBD isolate with T41 & returned about 66% total cannabinoids. Does anyone know how to separate the unknowns from the cannabinoids? Better yet does anyone know what the unknowns are?
I attempted distillation with 160c condenser just to return the same potency.
Did you do a lipid check on the isolate?
Better yet. Use a different catalyst and all your cannabinoids will stay visible. No mystery oil for your clients. And no wasted noids for you. Win win.
BTW I’d love to know too. I think everyone would.
this was a cbd isolate/T41(I believe) conversion from CBDokie circa June, COA is from Dr. Jebril. He said before he thinks the THCv is mislabeled. Someone else identified D10/6a,10a
![]() nobody knows the unknowns…
nobody knows the unknowns…
Don’t sell that shit lol. Eat it or feed it to your friends or something
Someone has to have done a mass to charge ratio & identified the structures, most universities have the equipment. I get a boutique test occasionally which reflects a lot of cannabicitran a little exo & a little d10.
I’m more concerned with isolating the d8 more than learning the unknowns. Raphael Mechoulam discovered most of them decades ago but the labs don’t test for them. My theory is that it’s dominantly CBD hydroxy quinone or HU331, have yet to find a lab with a standard to analyze it though.
or maybe just don’t use T41? there are several way cleaner catalysts.
T41 was just an example. Plain carbon would be cleaner. Any catalyst I’ve used still produces unknowns. Which one do you prefer?
PTSA, all day every day!
If you had to choose a base for neutralization—I’m guessing it wouldn’t be NaOH—any suggestions?
you don’t wanna sell partially converted purple liters for breast cancer awareness?
I thought that works better for d9. I like carbon & bentonite because it’s non-toxic. Water washes are my least favorite process.
![]() no thanks, my primary concern with this stuff is that the post-reaction work up is inadequate and there’s all kinds of catalysts, salts and the like still floating around the “isolate” or distillate.
no thanks, my primary concern with this stuff is that the post-reaction work up is inadequate and there’s all kinds of catalysts, salts and the like still floating around the “isolate” or distillate.
¯_(ツ)_/¯ purple distillate is real pretty tho
It’s not purple, if there’s enough azulene it will appear purple yet when the azulene is separated it is clear. If you throw a spoonful of sodium bicarbonate it will be purple though, a spoonful of citric acid it will be pink. The color is determined by the ph even in absence of a catalyst.
i didn’t choose bicarbonate, bicarbonate chose me
mine, too and my wife isn’t happy with it ![]()
I always wondered how that can be, because solvents other than water don’t have “pH”.
Things are still affected by the potential hydrogen even in the absence of water. Methods of analyzing pH are limited. It’s still possible to extract a pH analysis from something anhydrous by using water just as something anhydrous can be affected by pH.
you’re going too far there for my taste.
yes, acids can dissolve in solvents other than water, but pH is defined as the concentration of Hydronium ions in water.
also, PTSA dissolved in an alkane or arene for example might dissociate or protonate another dissolved compound, but that’s not really “pH”, while it may account for some of the colour phenomena observed in isomerizations.
my current understanding is that most of the coloured compounds are formed by interacting with oxygen, and acid concentration is more like a secondary factor.
Oh jeez, didn’t realize it would come down to semantics, let me retract the term pH and say acids & bases. Distill with an acid in the flask & you get acidic distillate. You can test this by mixing distilled water with your distillate to find what its pH is. Things are still acidic or basic even when theres no water, I call it pH.