Hello fellow psychonauts and cannabis enthusiasts alike,
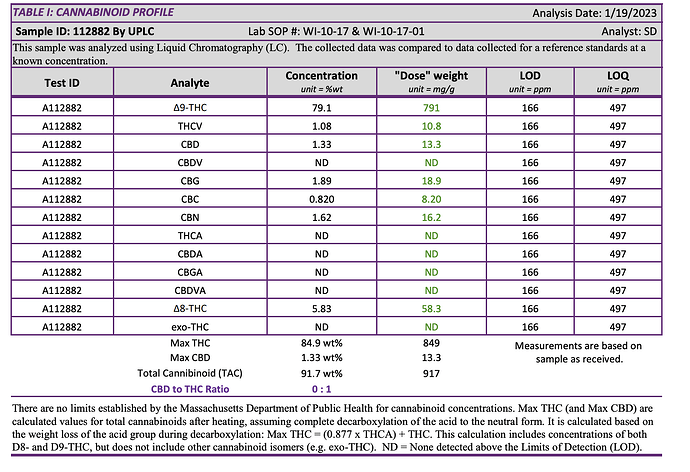
Like many other operators on this forum, I am having an issue with CO2 extracted crude isomerizing into other forms of THC during various steps in the process, particularly distillation. We are producing beautiful, clear, potent distillate, but anywhere from 3-15% of is comprised of delta 8, delta 10, delta 10a/6a, or exo-thc, along with other “unknowns”. It is consistent that at least 3-6% of our distillate is made up of these isomers, and we are trying to figure out where and how this is happening.
We see the presence of these isomers in smaller amounts (<2%) even in the crude, and as the crude goes through the winterization process, this number increases just from the heat of the water bath during rotary evaporation. Finally, the number really jumps up from short-path distillation. We were getting distillate with up to 9% Delta 8 THC after some short path runs.
We also run a Root Science wiped film unit. Part of their original SOP includes cooking the oil on a hot plate at 150C for 4 hours to ensure full decarb and devolatilize the oil. We purchased the short path set up to do this process under vacuum and have more control over the devol. Interestingly, the oil run through the short path has produced higher levels of THC isomers than the oil cooked on the hot plate exposed to ambient atmosphere…
From reading various posts on Future4200 including Isomerization of Co2 extracted Crude, Isomerization to delta 8 from heat alone?, and What happened to my THC content? Lab says conversion into delta10. How?, it seems that the acidity of the crude, plus high temperatures, enhanced by reactivity under vacuum are responsible for this uncontrolled isomerization. Although this change can happen with a variety of crude stocks, it seems to me that it is more prevalent with CO2 crude, for whatever reason. Possibly the inclusion of naturally occurring plant fatty acids that are extracted with the non-selective nature of the supercritical CO2 solvent characteristics?
Testing the acidity of crude, or distillate for that matter, is a tricky one. Dissolving it in ethanol and testing the ethanol is not accurate because testing pH in ethanol is itself not a good representation. We have tried dissolving the oil in heptane, washing against water, and testing the water afterwards, noting that the water does indeed become more acidic, although this is a relative measurement at best. Nonetheless, we do believe our CO2 oil is acidic to some extent. We have tried to utilize 3Å molecular sieves in our extractor to catch any water and prevent carbonic acid from forming. We utilize other filter media directly in our extractor to enhance the quality of our crude and pull out some waxes.
If there are other CO2 operators on here, have you encountered this? To what extent?
What is more responsible for isomerization of delta 9 to its analogues: acidity, or heat?
Does vacuum enhance this chemical reaction, or slow it down?
Most importantly, is there a way to treat this distillate and reverse the isomerization?
We are open to engaging with a consultant who has had experience on this topic, especially if they have experience with CO2 extraction.
COA’s and Chromatograms included below for reference:
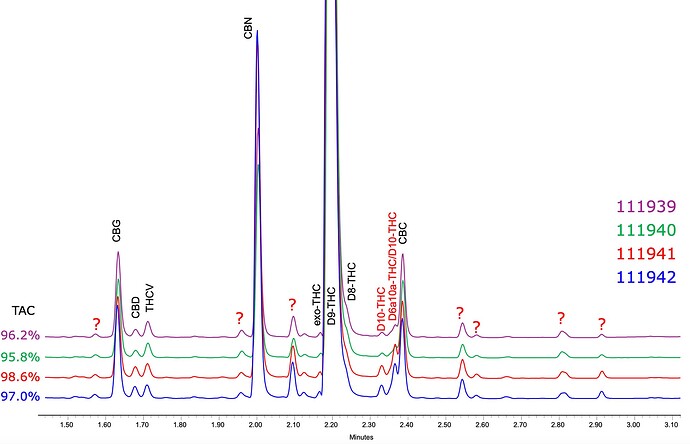
This is a batch run through the short path:
A deeper look at the same batch:
230206_Distillate Isomers Peak identification:quantification.pdf (317.7 KB)
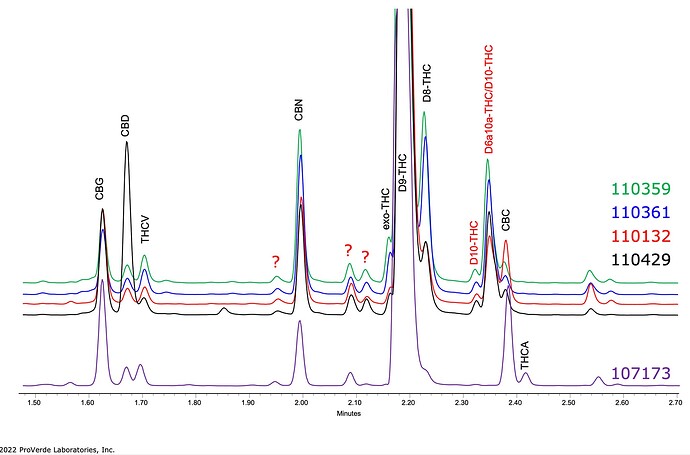
A few other batches: