Do any of the scientists using HPLC see small amounts of d9THC ( .1-.3% ) in CBD calibration standards purchased from Restek, Lipomed Cerilliant etc.
Hugh
SRI
Personally, I have only purchased the cannabinoid mix with d9-THC, CBD, and CBN from multiple vendors. But I am sure some of these single analytes have trace levels of other cannabinoids in them. I have never really delved into checking our standards for other trace level cannabinoids. Most of the time we dilute down to 25 ppm for a check after receiving the standard. So we would dilute out any trace level cannabinoids at the same time…
If you see this with a GC, it indicates that you got liner activity, and need to replace it.
Are you getting the detection across multiple instruments?
Since you are getting this detection across multiple standards with different manufacturers I would suspect something related to the instrument, sample prep, or a contaminated standard by an analyst.
There should be a COA for the standard from the manufacturer as well.
Liner contamination should show up in solvent blanks as well. No?
Here I’m dealing with a specific type of contamination, where the contaminant catalyses CBD–> d9 conversion into the heated liner. One may see some .xx" of d9 in CBD isolate then… ![]()
So far, I believe the most likely culprits are the classical auto-injectors, which are too brutal to my opinion, and push pieces of liner into the septa. Those pieces of septa may further develop catalitic activity upon thermal alteration. But dirty samples can be responsible as well.
I never had such issues before switching to autosamplers, despites using same liners for lots of samples. Hence it should be less likely to be encountered with a SRI.
Oh boy…
could it just be that the auto injector, by stacking runs back to back in a manner that is hard to achieve manually (ime) is allowing the accumulation of said contaminant in the liner?
Or even just the greater number of samples you’re able to push through is making this problem evident?
This, no I don’t think.
The main difference between auto and manual is the way the sample is injected. It is much smoother by hand. The autosampler really punch the septum. The agilent people told me there is no way to make it slower. ![]()
This yes. It is likely one other reason.
More samples, and more diversity.
Thanks for the input/feedback.
The standard COAs claim 99% but don’t identify the missing 1%.
I can’t distinguish between trace d9THC ( .1-.4% ) in the standard ( if it exists ) and isomerization ocurring in the GC. I don’t use a liner. This is on-column injection. I notice a different profile of d8,d9 depending on the column so I am leaning toward the isomerization in the GC explanation.
I’m told GC columns are generally acidic surfaces and there is also heat. If I had an HPLC handy I would test the standards and look for trace d8/d9.
Is anyone aware of some reagent which prevents isomerization as I might be able to add this to the injected sample
Hugh
SRI
Using LC here, not noticed any THC in our CBD standards from restek or absolute standards. Ive asked where their standards come from and they are evidently synthesizing (as in not plant derived) so not much of a chance of plant based contamination.
I could see the possibility of isomerization happening in the hot GC column though.
My guess is a catalyst contaminated column
Less likely at SRI headquarters than the rest of the GCs run by folks hanging out here I would wager
Yes,
I also see d9-THC in Restek D8 standards…
Same here. I actually see a decent amount of “other stuff” in restek standards. I thought I was losing it at first. This is with GC. I get anal with my testing.
Solidified my suspicions (and is good practice anyway) by making sure I have a good, no solvent injection baseline. That will tell you if there are any contaminates. Then pure solvent injection (no internal standard, as pure as possible). Besides solvent spike, should be no other peaks. Then solvent with internal standard, all good there. Then moved onto just an injection from the calibration standard, and there they were. They don’t appear in cereliant. This to me concludes it is in the standard itself from restek. A single component standard has them as well.
My thought is that when you buy the standard, they are certifiably stating that there is X amount in Y. 1000ng per uL of CBD for example. You can see the details from restek on the samples and their methodology if you put in the lot numbers from your sample on their website. This doesn’t mean that the sample is PURE. Just that it has x amount of Y. In my experience, I have not had any contaminants directly near or seem to interfere with the target compounds calibration, and the target compound area is the “same” across different batches of standards. BUT= when comparing standards from restek to Cerilliant, we get different area counts. That’s a whole other discussion though.
Have you tried to inject (almost pure) CBD as well ?
This is the most straightforward way to ascertain the occurence of liner activity.
Oh of course. and you are correct. We have had a contaminated column actually that caused CBD → D8 conversion during testing in the GC. The acids used for conversions was not fully neutralized prior to testing the sample, and altered the columns chemistry. When testing CBD standard we would get a reduced peak then a smaller peak that eluded at d8 RT. Couldn’t revive the column and had to purchase a new one. This is one of the reasons I do not purchase the premix standards anymore. Column and equipment health is the the core basis of good analytics, followed by methodology IMO.
So yes we have experienced both scenario’s in this thread. I want to add the contamination seen in the restek standards are fairly minute. Back when we were using the premix standard from restek, there is always 4 small peaks between CBD and D9.
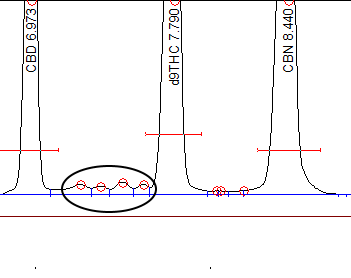
The importance of this topic is that we ( SRI ) were concerned that isomerization of the sample injected into a GC was inevitable because of the heat and potential activity of the GC column. While the amount of isomerization was small ( .1-.4% ), this still rendered the GC potentially un-useable for CBD isolate analysis where the goal was to measure less than .3% for d9THC.
The only way we could test for isomerization was to inject a “supposedly” pure CBD standard from Restek, Lipomed, Cerilliant or other standard supplier. Injecting an extract of CBD isolate itself was not illuminating because there was no way of knowing if there actually was some small amount of d8/d9 in the isolate. We initially thought there was some inherent isomerization because we consistently saw .1-.4% d8/d9 in the “supposedly” pure standards.
I asked Restek to use HPLC to look for d8/d9 in their CBD standard and they graciously agreed.
They saw .02% of various cannabinoids, but nowhere close to .1-.4%. We bought two ampules of the same lot# and tested one ampule immediately on receipt. We did the same for a Lipomed standard, but did not get an HPLC analysis. We also had a Lipomed ampule in the fridge at 5C for about a year and broke that ampule at the same time for comparison.
The chromatograms are shown here.
isomerization.pdf (1.1 MB)
The takeaway from all this is that the GC does not inherently isomerize the sample. The isomerization occurs in the standard even if stored cold. There may be exceptions to this if the column has been acidified but this is a separate issue which we are hoping to resolve by finding a way of re-conditioning a column which has been acidified. Any suggestions for a “magic” reagent for this purpose would be appreciated.
Hugh
SRI
This you could actually easily checked by injecting CBD isolate samples into a clean GC system. ![]()
Besides CBD, the only peak that should show up would be CBDv an another unknown in similar proportion. Plus CBD isolates can be further purified from trace CBDv by recrystalization at room temp.
Thanks.
How can you be sure the CBD isolate does not really contain small amounts ( .1-.4% ) of d8/d9.
This whole issue was started because a CBD isolate sent in for a test showed d8/d9 when the COA from the 3rd party said no d8/d9 detected. The suspicion at the time was that the GC was causing the isomerization, but the data I presented here indicates the GC does not isomerize, the d8/d9 is really in the sample.
Hugh
By injecting a concentrated sample in a clean system. With a l.d. below .1%, if one sees no d9 peak, one can be sure there is not any d9 in there.
D9 does not co crystalize with CBD.
Some could be trapped in inclusions, still.
Hence the interest of repurifying almost pure isolate several times, at least until any color in solution is gone.