But you would never write down that it’s because it degradates in dangerous compounds right? Consumers are used to think a couple of extra days after expiration of a product is not a big deal, specially if it’s an smokable, they could think its just a quality standard issue more than a health issue, and if they are pot ungry and they have just some rest of it after some bears, don’t you think they would say wtf let’s smoke this…
I would do it even knowing this.
I got a sample of HHC-o-acetate from the lovely @anon32743824 and I must say it is far superior to the THC-o-acetate I have tried from other vendors. It’s water clear, odorless, and about the same viscosity as THC-o-acetate but gives me much less lung irritation. I get a nice head high kind of like HHC, but slightly stronger and longer lasting. It is great on its own, but I might add some other noids and terps to take it to the next level.
Cough cough. Samples @anon32743824 ;0
That was the thc o acetate not HHC O acetate
Got me all excited
D8 or d9?
Sounds like d8
Not all hhc is created equal
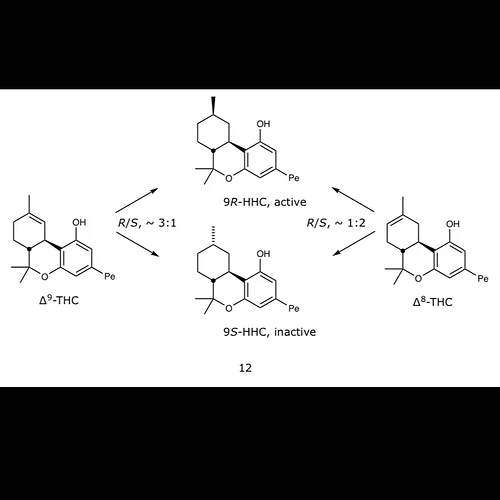
D8 hhc makes a 3:1 mixture (3 parts inactive to 1 parts active hhc)
D9 makes a 2:1 mixture (2 parts active to 1 part inactive hhc)
This is in the old literature, you definitely want hhc from d9
My mistake, you mentioned both so I wasn’t sure which you sent. Is the thc-o-acetate I have d8 or d9?
Delta 8
I hear the same applies to d9CBN but haven’t heard anyone else talking about it.
Close. It’s 3:1 in favor of the good stuff from d9 and 1:2 for d8. As far as one can trust Mechoulam’s assignment.
Entirely dependant on catalyst. Presenting those ratios as fact is pretty misleading if whoever reads it doesn’t know better.
Not really, all hydrogenations use a metal and an h2 source
Youre literally arguing with Mechoulam who used h2 gas and pd/c
This is from the old literature
I understand that its from old literature. I get the impression you’ve not run very many of these hydrogenations with your own two hands? And I don’t mean any offense by that - but once you actually run a number of these reactions you’ll pretty obviously see inconsistencies with what you’re regurgitating from literature and then the actual reality on the bench. Selectivity DOES have something to do with starting material, like you’re suggesting, but it has a lot MORE to do with what specific catalyst and conditions you run. Seriously, run the hydrogenation a couple hundred times a couple dozen different ways and you’ll see where I’m coming from.
I’m not arguing with the mighty mighty Mechoulam, but…its not like every statement and conclusion and experiment he’s ever put out has been infallible or perfectly accurate. Use other work as a guide, but don’t blindly follow what you haven’t seen with your own two eyes in your own lab.
Do you have any test results to prove anything you’re claiming?
I’m cant just hand over research - I’m already offering free insight here. Buy the same catalyst from 5 different vendors and run the same conditions and observe the variability, then run 5 different catalysts at 5 different loadings, in 5 different solvents. Now vary temperature and concentration. Do the chemistry with your own two hands and crunch the numbers. I promise you I’ve run this reaction time and time again - I don’t know how many times you’ve done this work if at all, but if you haven’t done these things for yourself then you’re putting a lot of faith in someone else’s work and disadvantaging yourself and anyone who takes what you’re saying as fact.
All I’m trying to illustrate for you is that there is much much more to catalysis than breaking some reaction dynamics into some ratios and claiming that’s how it always is and will be. Its not insightful mechanistically, and is a facile simplification of what is actually a quite intricate reaction dynamic. You’ve got to run the chemistry for yourself - some of it will agree with literature, some of it will not, some of it isn’t in literature, but you will absolutely realize there is more to the story than some fixed ratios.
inappropriate - edited
So you don’t have any analytics to prove what you’re saying basically?
You never even mentioned trying d8 vs d9 which makes me wonder if you yourself has even tried it, not being a dick im skeptical of anything someone says on the internet without proof. I dont know of a lab that can even quantify the 2 isomer of HHC which is why I was asking if you’d actually had it quantified or were guessing based off HPLC graphs
I didn’t say I don’t have analytics. I said I’m not going to just show off hard earned research just because you requested it.
No, I don’t have an HHC standard. I’m more or less beholden to using area count because I don’t have a standard and I don’t have a chiral column, like pretty much everyone else.
I’m sorry that in my extremely brief description of how to optimize this chemistry I didn’t explicitly state varying d8 and d9 starting materials. I honestly wasn’t writing an exhaustive research program for you to follow - I was illustrating some of the many parameters you need to vary along the way before jumping to a conclusion.
I didn’t come here to be like “I know sooo much about this and you don’t.” I came here to say thats a oversimplified way to present that information and it overlooks the true complexity of the catalysis.
So you’re basing your finding off of area on an hplc graph without even using a chiral column or having a standard? That might be good enough for you but it definetly isn’t for me
Thank you for your input, I’ve had bad experiences with relying purely on hplc which is why it’s hard for me to believe what you’re saying. Hopefully @kcalabs will be able to quantify them soon as they’re really the only lab I trust at this point (I wouldn’t even trust in-house analytics)