Has anyone tried the acetic anhydride reaction with less acetic? The stoichiometric ratio according to my calculations is 2:1 acetic anhydride:D8 in the given SOP. But only 1/2 of the acetic anhydride would be consumed. That would mean lots of left over acetic anhydride in the end.
From what I’ve read 2 equivalents is necessary to achieve full conversion. If you don’t need full conversion for your application I’m sure you could use less.
Ok. I am getting TLC setup to see if its necessary in my lab. My math tells me its not necessary but I won’t argue with experience and hard data.
I wonder, are people really churning out pure THC-o with this SOP? I’m very surprised the workup doesn’t include a bicarbonate wash and a distillation. I’d believe that your product could be reasonably pure if you used very pure feedstock, but just waiting until TLC shows full conversion and then evaporating until vinegar smell is gone just doesn’t feel right to me. I’d love to see a COA of THC-o made with this SOP.
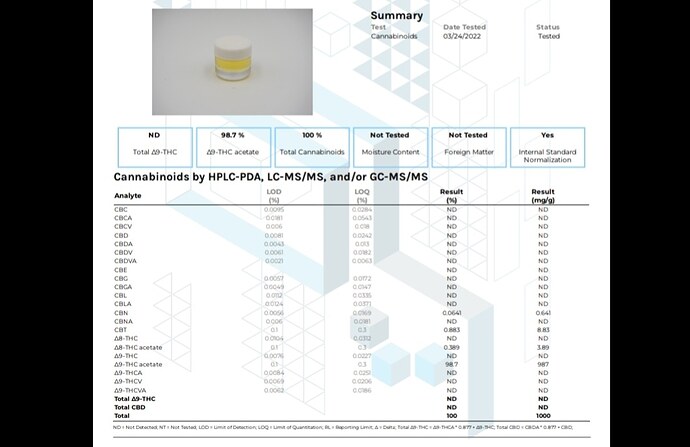
My sop makes pure thco
Confirmed by gcms by kca
No thc at all and pure cannabinoids
I can tell you there’s a trick to get it without using an amine
You need a catalyst to get full conversion
i’m using anhydride and a weak base catalyst. Is that what you are using as well? Also are you starting with 99% D8?
I distill after evaporating. I am starting with 93% D8 and getting back 88% THCO
I’m starting with d9
It sounds like you’re using a different sop then me
I started with 89% d9 and made what I posted above
Would you be willing to share your SOP with us?
your not using DMAP by any chance are you? I guess probably not since you said you don’t use an amine. I just ask since I’ve heard DMAP be praised for it’s ability to catalyze esterifications at room temp.
Amines are only used for hard to acetylate alcohols
Thc/cbd isn’t one of them
Has anyone tried this SOP or a similar one to make CBD-o-acetate? Apparently it doesn’t crystalize which makes me excited. I think I read that CBD can be diacetylated so it would be interesting to see experimental data if different amounts of AA make different mixtures of products.
You mean one needs another catalyst than an amine coumpound ? ![]()
I guess you are using sodium carbonate in ethyl acetate.
These organic reactions often require an excess of reagents to reach above 99% conversion.
Amine are nasty, and also hard to remove
So I avoid them
There’s literally no need when you can use something else that’s easier to remove
Someone walked me through the synthesis without acetic anhydride once, but it was in the middle of a convention floor and I was too stoned to follow. Pity.
Aspirin looks interesting
Seems counterintuitive, but I have yet to test this theory. Thanks for the reply.
I don’t really understand trying to work around acetic anhydride. Its cheap, it canonical/effective and its not particularly difficult to get ahold of. I wonder if people perceive some sort of hazard to it?