It may be exempt but I am pretty sure Cayman has to report back to the DEA who buys the standard.
This has to be one of the coolest websites on the entire internet I fuckin love this place
I may be missing something earlier but is there something wrong with using an HPLC for testing potency? Is it just access (legally) to standards?
Edit: potentially mitigated by the 1mg standards post above…
That’s what I’m thinking. Just access to a standard without a dea license.
No. Nothing wrong with using HPLC for these types of analytes.
Just make sure you don’t get hit with a conspiracy charge!
A very reputable analytics person on this site shared this with me when I asked how they were testing psilocin in their GC…
I basically employed the same method as I do for cannabinoïd, and it worked very well. I think psilocin was comming out before my internal standard (squalane).
There’s a huge chemical difference between psilocin and psilocybin, similarly to the difference between THC and THCa.
My colleague has psilocybin and psilocin HPLC potency testing up and running. DM me if you want to get something tested by him. We will be at MJBizCon if anyone wants to chat about it.
Normal phase or reverse?
Psilocin could work on straight phase HPLC although in my experience, an analyte with a free basic amine can cause issues with tailing and peak broadening. Psilocybin is way too polar to successfully go through a straight phase column, I would think.
Has anyone tried to just boof all samples? Works for me
A gentleman scholar
You ever boof DMT?
I think this could be accomplished on GC with a proper derivatization. Without knowing the exact arrow pushing mechanism of the dephosphorylation reaction I see several active sites that if derivatized would likely stop the reaction and create a heat stable analyte suitable for GC analysis.
What do you mean by active site? Like sites on the molecule that are charged?
find you a maldi/tof… lol
Active sites in terms of a site that can be derivatized, mainly looking at highly polar bonds/groups (-OH, NH).
For a lot of GC work derivatization targets are active hydrogens, which is a hydrogen attached to a much more electronegative atom.
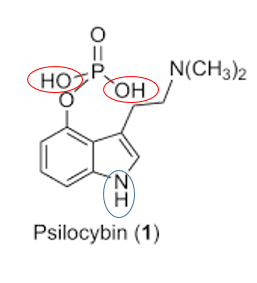
Hydroxyl (-OH) groups are usually suitable for this purpose but the N-H bond(s) can also work.
This looks promising.
For quantification, the GC/MS instrumentation was applied. Psilocin and psilocybin were silylated by the derivatization agent N-methyl-N-trimet-hylsilyltrifluoroacetamide.
Stríbrný J, Borovicka J, Sokol M. [Levels of psilocybin and psilocin in various types of mushrooms]. Soudni Lekarstvi. 2003 Jul;48(3):45-49. PMID: 14631713.
I think the “problem” with derivatization in general is that it introduces an additional step to your assay. Each individual step of an assay, be it the extraction, filtration, dilution, etc., introduces potential error and loss of accuracy. Derivatization can be quite error prone and often uses toxic/hazardous reagents.
In the particular case of psilocybin/psilocin I wouldn’t worry about psilocybin but instead make sure all psilocybin is converted to psilocin prior to analysis and only assay for psilocin.
Just like total THC in flower is THC + .88 x THCa, total psilocin would be psilocin + .72 x psilocybin. I think dephosphorylation is the way to go, giving yourself more options as to particular method of analysis.
Granted, there are other psilocybe alkaloids that you probably want to test for unless you’re aiming for pure psilocin.
