Here is a link to an article I wrote about terpenes and the cannabis industry and how R134a extraction equipment help make their end products more strain specific and contain more of theses valuable flavor and aroma compounds.
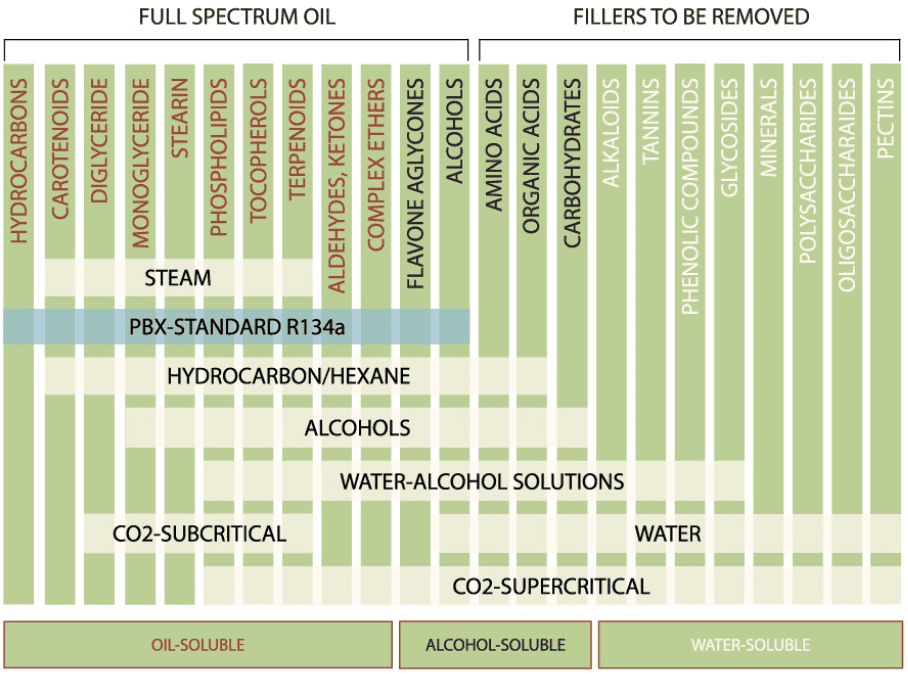
“In the diagram below a range of organic substances are shown and bars of the solubility of various solvents.” Hydrocarbons are not soluble in other hydrocarbons…? How do terpenes get extracted in butane/propane or any other hydrocarbon extraction than ![]()
Supercritical Co2 will also extract the things subcritical does so that bar needs extension. Depending on the hydrocarbon Water can dilute/mix with them as well. Alcohols dilutes and is used for extraction alkaloids and tanins as well… I can keep going but ill stop
Carotenoids included
Solubility isnt miscability ![]()
The whole concept of “solubility” tends to only be applicable to polar/ionic compounds/solvents.
Like WillBilly said, in organics there is miscibility instead…
Is that semantics around liquid/liquid vs liquid/solid interactions, or is there something physically different about those two?
Does one require compatible electrochemical interaction between the constituents and the other merely the lack of unfavorable interactions?!?
Also when you are talking about reduced solubility with CO2 don’t forget that you have recurring loop where the solvent recirculates over the biomass… so the fact that CO2 has “reduced solubility” means you only need a longer extraction.
Is it possible to just scale up a C02 system to run more “solvent” to speed up the process?
In theory, yes… the pump size (mass of CO2/hour) is what you want to look at. Some like Thar Process units have like 4-5x the flow of an IES or Apeks unit.
Anybody ever tested a r134a extract for residuals?
The entourage effect is a lie. And a terp is a terp. If you are looking to extract terpense may I suggest a different biomass.
@TheGratefulPhil did we ever put the turd thru the gcms?
Entirely
We can argue meaningless semantics all day, If you would like to get that technical lets. The dermal sheath of glandular trichomes of Cannabis consists of cuticle and a subcuticular wall - which are not liquids. Further more they require a solvent with similar polarity to “unsheathe” (quotations in place for any more semantics use the verb you’d prefer.)
In short you’d be correct if the terpenes(“liquid”) i am referring to was obtainable/extracted without the use of a non polar solvent. Considering Hydrocarbon solvents(yes there are others that can be used like ethanol since i need to defend unsaid things) are needed to obtain so id argue solubility everyday.
The picture is in reference to extraction, not mixing when post processed. Only liquid terpenes im aware of require a solvent and some extraction method whether it be steam, hydrocarbons, ethanol…
Can it be attained without? yes, is it efficient or tasty? no. So in short irrelevant to our extractions as those are key to quality product/profitability.
After further thought, it isnt semantics. It indefinately is solubility considering the topic is of extraction from plant material and not mixing post processed materials.
I don’t think so, but the stuff was pretty flowable I’d have to guess it did indeed have some r134a left around.
A lot of hydrocarbon extractions in calis early legal phases in 2018 were r134a extracted, they were tested but im sure not for what was needed to be tested.
Considering r134a has a boiling point closer to propane than butane id argue the opposite. Indefinitely less likely to have more r134a remaining than butane if post processed in the same manner. As for any other constitutes in the r134a(if any), i can not attest.
Thems the ones I’m thinking about. I’ve never seen r134a evaporate completely clean, even on a hot day, when doing ac work.
Hey, @cyclopath, do you remember when we went to a facility to see the prototype of this system? I do! Good times… ![]()
I sincerely hope you guys have got it making products at least 100X cleaner than we saw at that demonstration, @DarrilDevil! I’m guessing you have! Kudos on that optimization work and on this nice little article! ![]()
I’m not sure I’ve ever heard this argument, specifically, @anon64373531 @TheWillBilly… I just know that miscibility is defined by two liquids that are highly soluble (often “infinitely” soluble) in one another. Id est, two solvents are miscible if they combine into a single homogeneous liquid phase… EDIT: at all proportions.
I think what you are writing about here, @anon64373531, is disassociation versus dissolution… Ionic compounds can both dissolve AND disassociate into cations & anions in polar solvents. These two properties are intertwined, but only insofar as disassociation requires a substance to have already dissolved. The inverse is NOT true: Plenty of non-ionic things dissolve in each other without disassociating to form electrolyte solutions… and even ionic things can usually dissolve MORE than they can disassociate at a given set of conditions.
I’d have to disagree.
This was crude straight out of the pot and the system did not recover to a super deep vacuum as far as the person who manufactured it told me.
I’ve seen residual butane as high as 10,000ppm in basically unpurged crude.
Boiling point of -20C so it seems outlandish, but if I had to guess it still had some in there.
Wish I would have run it in the GCMS to know for certain!
I tend to lend myself more than enough rope to hang with organic chem. This is usually one of those places.
I think of “solubility” being driven by positive ionic interactions. Salting out works by replacing that interaction with a more favorable one leading to precipitation.
I likewise think of organic solutions as miscible rather than solvated, and organics “squeezing out” polar compounds due to lack of favorable interactions. For me a hydrocarbon extraction is simply breaking up of the trichs and sweeping away of the miscibles.
I, being of wide enough mouth and with habit of stowing feet there, am always open to correction.