Hey Yall semi new to the game we just started extracting a few weeks ago and since then have revised SOP a number of times. Yesterday after my initial soak i ran some solvent through then hit a second soak but this time turned on my pump to build some pressure and soaked at 100psi vs the 50/60 I normally have with the pump off. I seem to be getting better yields with the increased pressure but normally my final product is a nice terp sugar almost like kinetic sand with a sauce but after adding the pressure to the soak yesterday what i got was more like a cross between crumble and sugar. still smells amazing and the color is about the same orange hue it always is we run dry ice through an injection coil and fresh frozen material in frozen columns so I dont think we picked up any extra waxes or anything. What do yall think is adding pressure to a soak helping or is it causing me to pick up undesirables? Just trying new things to see what works right now looking for some advice to make sure im on the right track. Ignore the color I was under HPS lights using a filter in a darker corner so it looks dark but its pure gold.
I just accidentally extracted some material with room temp butane at about 120 psi for 24 hours and sent the extract thru bentonite.
I certainly did pick up all the waxes available and all the carotenoids… lol.
Yea n butane the product goes off for testing next week.
First post and it was all just a processing error i feel dumb now. Left it on half vac overnight so all the trapped bubbles changed the finish. After pulling full vac on it now its starting to look like it should.
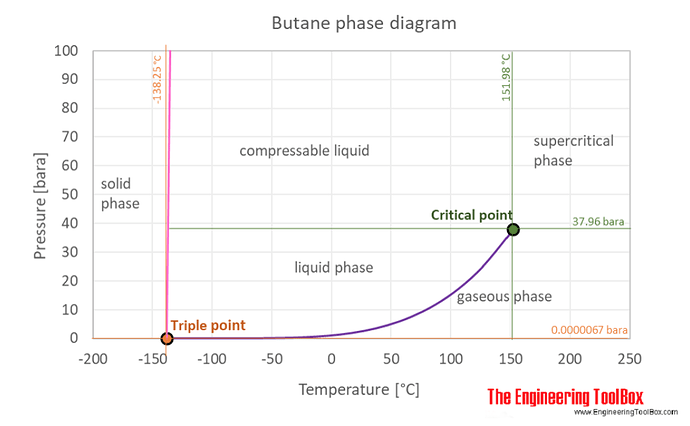
Can someone explain that phase diagram a bit?
The way im reading it since im keeping the solvent sub zero increasing the pressure shouldn’t cause an issue.
As far as the phase diagram above, it really just shows that you are squarely in the liquid region. If you are keeping the system cold, you will stay in the liquid phase at most any pressure you are working at. The ‘compressible liquid’ area is up over 500 PSI, so not an issue with this extraction. The diagram does not give any real info as to how effective the extraction will be.
Generally, you can assume that an extraction at a higher pressure (compared with an extraction of all the same parameters) will perform a more ‘complete’ extraction. The effect would be similar to performing an extraction at an elevated temperature, although I imagine pressure will have much less of an effect on the end product compared with changing the temperature. Pressure is a less sensitive variable with respect to extraction. There are 2 reasons I can think of off hand as to why the extraction would be more ‘complete’.
First (and only reason applicable here) is simply space-filling. Imagine your material column filled with gas and material. If you were to dial up the pressure at this point, the butane will get ‘squeezed’ into the material with more force, more thoroughly soaking it. The extra pressure forces the gas anywhere it can move into, and squeezing a bit more into the material is the only place the gas can really go.
The second reason is due to fluctuations in the actual molecular structure of the gas. As pressure is dialed up, the structure of butane/propane can get distorted, flopping about through different conformations which can effectively increase the polarity of the molecule for a short time. I have not run numbers on this, but I imagine this would not be a major effect until you get near 1000 PSI (or maybe ~500 PSI, aka the compressible liquid area of the phase diagram). As a interesting note, this distortion is a key principle in sub and supercritical CO2 extraction, one of the reasons extraction pressures are soooo high in CO2 extraction.
BUT, this is not really beneficial for what most of us operators are trying to do. The vast majority of stuff we want to extract is on the outer surface of the material (in trichromes), so increasing pressure and getting into the middle of the plant material and into the actual cells of the plant is really just extracting unwanted compounds.
How do we rig up a supercritical butane or propane extraction? I’m assuming we use a co2 extractor with an inert gas sweep to remove oxygen💣
Anyone ever done this?
I think some people have done supercritical propane on here. I remember @Roguelab was toying with the idea? Although I think both of us constantly think about weird shit we wanna do… Did you ever get that ethane extractor going? ![]()
![]()
There’s certainly nothing more fun than finding something flammable, then putting even more of it in a tube, then pressurizing it into instability.
and then making drugs!!!
You talk about something here that I had wondered myself for a long time, so thank you first of all for touching on it, and second of all I have a follow-up question.
My idea was that high-pressures (such as super-critical) literally cause the molecule to change its structure such that it affects where the positive and negative charges may be, thus affecting it’s polarity. And it is manipulation of this that affects solubility with respect to polar/non-polar compounds.
Am I on the right track? I’m really trying to round out my understanding of solubility as much as possible and fill in any gaps I might have.
okay on further reading I don’t believe the shape of the co2 molecule is changing in response to supercritical pressure.
Found the place for this. I have no interest in BHO anymore but for those who do and are wondering about additonal pressure, here’s what i found when i did pressurize, it produced a unique result, the color was perfect. I didn’t take any other pictures after this, only after initial wash and first evap, never after purge and never final product but it was turned into beautiful light amber-honey oil.
The PSI was pushed to 150psi in a stainless extraction column designed for a closed loop system, the column had ball-valves on top and bottom, i used compressed air to add pressure, it was i guess a weird mixture of an open/closed blast as it was not looped but was closed off so it wasn’t technically an open blast? Don’t know the specifics here.
This was a fast wash, tane in, pressure in, tane out.
Here is the result:
FOR ANYONE WHO IS PRESSURIZING THEIR EQUIPMENT WHEN USING FLAMMABLE GASES, YOU ARE CREATING A BOMB AND IT WILL KILL YOU IF IT GOES OFF, THIS ISN’T SOMETHING TO BE TAKEN LIGHTLY
Wtf lol aint no way
DON’T USE COMPRESSED AIR FROM A COMPRESSOR. FOR THE LOVE OF YOUR LIFE AND TO BE SAFE PLEASE USE AN INERT GAS; SUCH AS: NITROGEN, ARGON OR HELIUM.
No helium. ![]()
- shit is currently finite
- helium will find a way to leak due to molecule size
Least till we get production from hydrogen working…
Argon and helium wtf.
Burn the land and boil the sea,
You cant take the sky from me
Just saying to use inert gasses but mainly nitrogen.
Using compressed air is careless and dangerous.
Had no clue about the helium thing being prone to leaks.
Yeah argon but I think it’s like a super last resort.
If you’re competent it won’t kill you. I’m just fine. Although i would never recommend anyone to do it lol.