@envee It’s not about being competent. Oxygen plus hydrocarbons in your system should always be avoided
but… but… i like making bombs
Supercritical hydrocarbons are hot hot hot…
I wouldn’t go above 200psi or your gonna start burning the bio while extracting it.
Our now granted extraction patent is focused on high pressure hydrocarbon extraction which has many benefits when dialed in properly.
Given the current legal status of cannabis, was your patent granted for extracting hemp at temp?
lol what? Never noticed that at 150.
It is for any bio that contains a product that dissolves into a hydrocarbon solvent. It is our method and device that is patented.
150psi is good… But over 200 it starts getting hotter at a faster rate per psi than below 200psi and you can definitely cook the bio. Goes in green, comes out dark brown/black.
This is also assuming you are using our process which does not utilize nitrogen or other atmospheric gases.
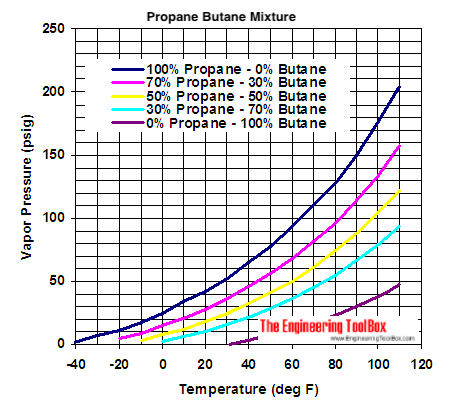
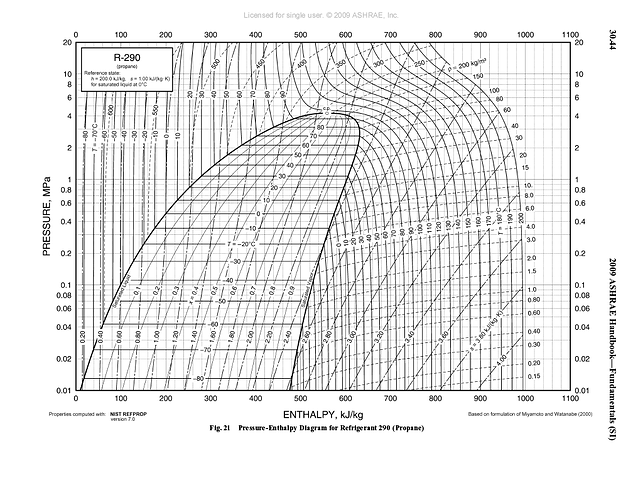
the above doesn’t extend past 200psi, but you can certainly see the trend @Zack_illuminated alludes to in the shape of the curve…
Yep. Even achieving 50 psi without N2 is problematic when running Fresh Frozen with butane only.
Biomass not burned, but nevertheless quite unhappy about the process…(see chart above)
![]()
Oh you definitely can burn it (just doesn’t oxidize)… we did a run at 320psi with propane one day just to see. Went in dry and bright green, came out dark brown with black spots.
Edit: I suppose it wasn’t truly “burnt” but it makes everything into poop, including the extract. But it got EVERYTHING out… Interested in trying non-cannabis products that can handle higher heat.
Any chance you could explain what we’re looking at here?
Enthalpy is not going to mean much to many of our readers…pascals may also confuse some.
Don’t believe I’ve ever gone above 35 psi when pushing through with nitro.
Processing: 8E38D072-A83A-489C-A2CD-3A390A9F1DA1.jpeg…
Am I missing why the OP is working at 100?
Ah, my bad. So if higher pressure inherently equals high temps, is this any different than just running without cooling your material column, like many do for shatter?
Lots of data on a NIST chart… could have an entire class on just this one topic.
In general though, enthalpy is total heat content of the solvent and 1 Megapascal = 145.038psi.
The curve is the vapor-liquid line where it transitions between states. On the far left is 100% liquid and potentially sub-cooled or very hot at various pressures, in between the two its going from liquid to vapor (evaporation) or vapor to liquid (condensing) and cannot be any temperature other than what it’s pressure determines, and on the far right it is 100% vapor and also potentially superheated and can be very hot at low pressure.
The enthalpy lines are perfectly vertical from top to bottom going horizontally as enthalpy increases and pressure lines are perfectly horizontal from side to side going vertically as pressure increases.
I grossly overlooked that we were talking about the temperature of the gas.
When I said 150PSI, I was talking about the pressure I moved with. 150 PSI gas, from its own pressure/temperature, definitely gives me some pucker.
Why use pressure when you can just lower the temp? Just factor in that the material will need to have heat removed by the solvent at the beginning of injection. If you want to increase the yield just increase solvent ratio to the material. I generally run at 20psi nitrogen.