Mmm egg fart dabs
i computer automated my closed loop, so having the variation in mole fraction butane/propane is something that is nearly impossible to program without some advanced measuring device for said mole fraction. with a single liquid i can use physical constants to determine the right variables.
I’ve been working on automating my process, and I’ve been shopping for pneumatically actuated valves … can I ask how you automated? @hashmandu
Could you compensate for the variance if you created logic to calculate solvent ratios based on solvent temp and pressure? I think it could be doable if you’re only using 2 solvents. 3 or more would be impossible.
Wouldn’t be that hard.
Use a binary phase diagram for butane-propane mixtures. Determine composition based on temperature/pressure
Add the variable levels of nitrogen gas and atmosphere to that task and have it purge to the correct point every time using logic based on a 3d graph for Pressure, temp and molarity using a compound version of antoine’s or framentary experimental data. Not trying to be a dick, but i’d love to see you do it and back your claim up that it’s easy.
the problem is having the right data, physical constants, and accurate equations to estimate the the vapor pressure/ temp. There is also an accuracy issue as to where you measure temperature. in the Lab it’s not cut and dry like math. You can have multiple temperatures in a solvent tank and 1 pressure reading. the accuracy drops quickly with atmosphere, nitrogen l, and temperature fluctuations quickly with even just pure butane.
Kinda an odd coincidence fart smell only extracted when using 70/30.
Your evidence is anecdotal
it was over a thousand hours of study and engineering, lots of adderall, optimized capital outlay. I had to be 3 kinds of scientists and a business man. I did it myself and you can too just keep studying and researching. I can consult i promise it won’t be chad consulting i can save you a lot of time. i would want to charge for it though. my system runs all 4 tubes in succession with one button push. has fault and error handling. i programmed a touch screen for data collection, monitoring, parameter changes, manual control of entire system. and it’s c1d1
I agree it’s no easy task. The first thing I would do is scrap the nitrogen and create a recirculating pressure building loop. Temperature gradients can be eliminated with enough recirculation. I think it would take a lot more of an investment in process hardware to make it happen than logic writing wizardry.
The reality for most of us is that molarity is not a huge concern, having the system add the right amount of nitrogen and purge excess atmosphere without burping off excess solvent is the primary need for pressure temp ratio
Did I suggest otherwise? I’m merely saying 70/30 doesn’t always come as clean as you might hope from each supplier and a distillation doesn’t clean everything. I find it anecdotally odd that material which extracted favorably under ntane did not under 70/30 on multiple separate occasions as attempts were made to move to your anecdotally claimed ‘industry standard’. Do what you will with that information.
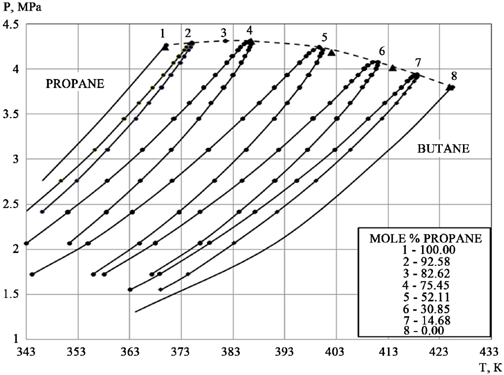
I dont know why there are multiple values for Y here for everything except pure butane and propane, but this would certainly cause ambiguity.
I’m assuming they are showing results during ramp up and down through those variables, where with the pure substances they likely used existing data.
Can I assume no nitrogen/atmosphere in the vessel where it is being measured?
If so, bet!
If you’re running your system efficiently you can. ![]()
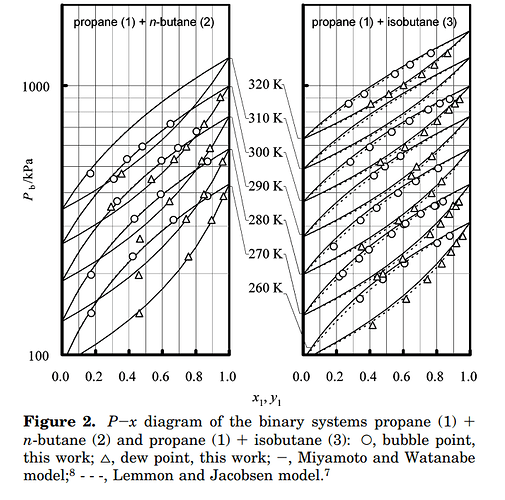
The intersection of these lines nearly prove to me that molarity cant be determined by temperature and pressure alone
On further reading it appears you are right. Bubble point is on the way up and dew point is on the way down. I’m guessing this is heat of vaporization gate keeping the phase trasition.
Why couldn’t you just do a simple flash calculation as above?
The determination of molarity of gas in a binary system in a flash vessel is a pretty standard calculation.
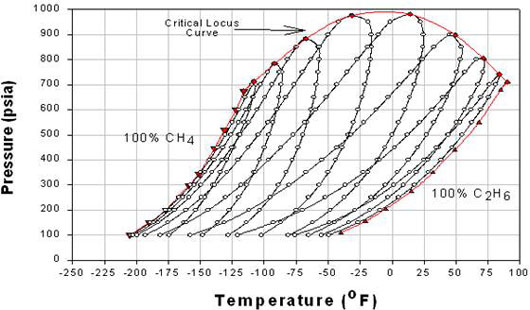
Even better–create a data model, like the one created here at several temperatures:
Then have your temperature and pressure transmitters send that information to a calculator and you have a rough estimate of your propane/butane molarities in the liquid & gas phase.
If you really want, I’m down to write out an example calculation.