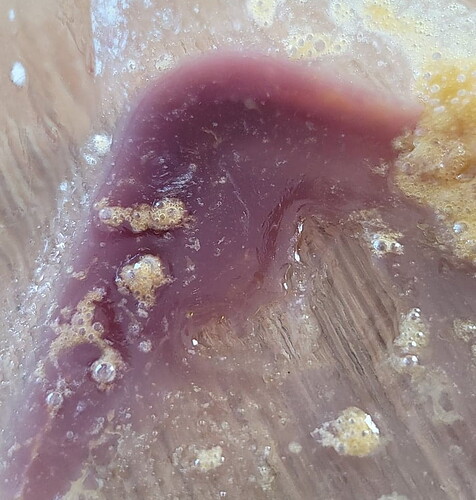
Def acidic i dont think by very much tho. It was hydro grown fed between the usual ph numbers so for sure below neutrality. It was some purp skurp that was not crc’d so yea probly the combination of that and the newly formed trichomes made a nice colored combination alil clear with some purp. Pink doesnt seem to abstract!
brb gonna light burn all my harvests and start a pink diamond fab
…basic…
Pink / red is from high pH, not low pH, which the latter actually tends to make it super clear or isomerize into d8 if it gets too acidic, along with the application of heat. Non-acidic molecules will also oxidize. THCa tends to be Red while CBDa tends to be Purple. From my experience, once a cannabinoid has oxidized (lost hydrogen) it will turn into a quinone and take on color.
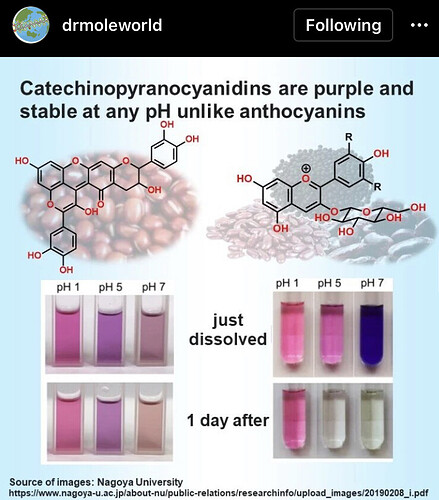
But then again, if it is Flavanoids (aka anthocyanins) then the pH only shifts its color from purple to blue to yellow to orange. Never will they be clear under any pH condition and always has a color to contribute.
If its a mixture then you are gonna have fun trying to figure out what is actually causing what. ![]()
Interesting, in this case I would lean more on flavanoids though… I do believe acidic anthocyanins are purple / red while more basic ones are blue. Could be washing them from the media at the end of the run. This is where it gets fun to actually figure out exactly what is causing what. W1 is acidic. Easy way to confirm is put 5g in 100g of water and put a pH strip in it.
Simple way to start is get some known molecule isolates and test in strong acid/base enviro… or just follow the data on the alkaline beam test. Same same. Data is everything.
Edit: You could also look at input / output cannabinoid pH to see what is going on in the column. More points to build a theses on.
Flavanoids during extraction:
You can actually see both colors you have in this one extract. The lighter tan/brown-purple along with the more rich and vivid purple. These are 100% flavanoids. The source was a partially frozen live run…
Simply by not being completely frozen, the mobile water then pulled the purple from the bio due to solubility.
Someone was just telling me this, and that blue thca is caused by a basic media
In your case I believe you are dealing with flavanoids but the OP I feel is dealing with pH. As if it were flavanoids it would be the same color from the time it left the CRC, whereas the other product was fine but then changed colors over time, that sounds like oxidation. Oxidation is accelerated by high pH.
It is all about that hydrogen molecule… High pH is an environment that wants to strip hydrogen to neutralize the basic enviro, as hydrogen on its own is acidic.
That’s actually how I pulled through the column, with the residual moisture from fresh frozen thawing
Yeah, that is totally falvanoids catching a ride in the water that is being pulled out during extraction… once in the extract they can be hard to remove. Maybe go with something more polar attractive / binding like silica or alumina.
Clear diamonds at one point and went to foggy white then to now basically pink thca, not sure how it happened pretty cool looking tho
I should clarify top pic was bho with w1 and the glass column was pentane and d9 with w1
d9 disty? Not as likely to have flavanoids but still possible. A fast hot pass can easily get a little bit of flavanoids in the main body. Co-distillation on the cannabinoids (not much different than steam distillation).
If it is a purged extract in pentane then its pretty much no different than if you ran bho through the column. Both pentane and butane are pretty non-polar, though pentane is more non-polar than butane.
I have done quite a bit of acid/base chemistry with damn near every cannabinoid just to see how they react. Lots of factors going on there… Funny to think at one point it was considered impossible to do a/b chem on cannabinoids.

Unfrozen live bio w/ lots of flavanoids + activated carbon media → shift that color from purple to blue.
He was speaking on the plants saps, not the pH of the solution(extract.) I can’t say i have read deeply enough into plant saps to see if feeding it around 5.5-6.0 ph really does keep it in that range or of it has some process like us humans where our body will “neutralize” i forget the correct terminology but pretty sure its related to our blood and how it balances itself out to a neutral PH( dont quote me on that its been years since i read into it when the alkaline fad came out) besides that 5.5-6 ph is most definitely acidic conditions.
Just because anthocyanins are in the mixture does not mean there is no other colored(colorless) pigments mixing with the extract appearing in a different manner. Levels of concentration of anthocyanins would have a large part to play as well. As stated it was open blasted- quick wash no soaks.
the “brb gonna light burn all my harvests” was kinda a joke i was making into how much time/money would be spent chasing something so pointless. If i wanted to color my dabs id just look into fruits and their compositions buy & mix the concentrated flavanoids/pigments w.e that people are already smoking in their concentrates lol. #sellingthepink100kconsults lol
BRB thawing now lol
I’d be curious to know the stability of the molecules might give more information on what it could be.
Pretty cool, decomposes to a colorless compound if left in light. easy extraction process as well