yes sir
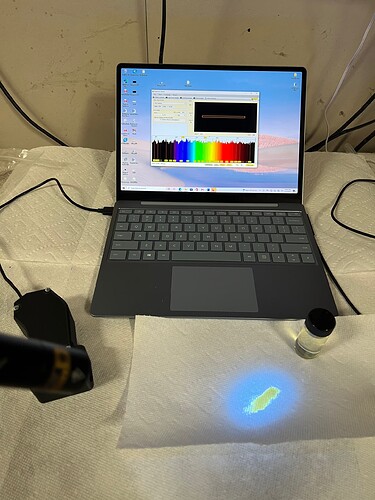
Shown here is the oil after a color remediation filtration. As you can see the oil is now a gold color in small amounts under white light.
Unlike earlier, under UV light the blue light from the carotenoids/xanthophyll is now the dominant source of fluorescent light color. It’s now difficult to tell if there is any red light being generated by the sample
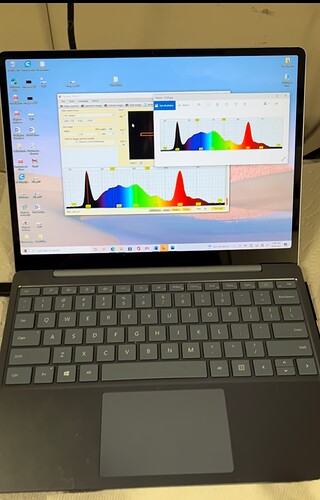
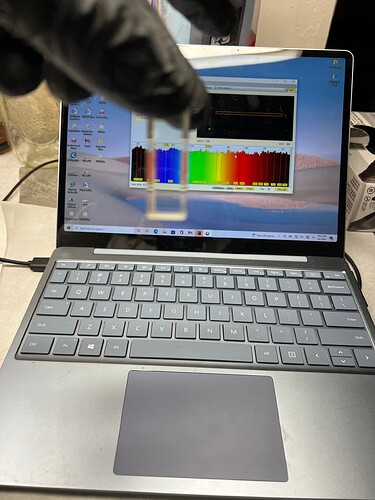
So we load the sample into the Pigment Tracker as shown in this video. The sensor can display the relative amount of chlorophyll and other pigments (carotenoids/xanthophyll) leftover after processing. This video also shows the data save feature which lets you take the sample data in jpeg form for future use.
The video will be added soon here is a screenshot after data collection:
What is the black peak?
The black peak on the far left is the excitation peak which is a UVa light. That peak is the high energy light the system uses to excite chlorophyll, which glows red in a double peak, and carotenoids which glow blue with a triple peak.
We are doing a 710 special where Pigment Tracker v2.0 kits are 10% off from 7/8-7/10. Total price is $400 plus tax and ship.
Happy holiday
Come check out the Pigment Tracker tonight at Xtractor Depot
Following the tech talks and throughout the evening I will be doing a free raffle at my table. Winner will be announced on instagram follow me there at Pigment_Tracker
This some really high caliber work. I dig the 3D printed housings, what kind of filament are you using to limit noise?
A perhaps interesting facet of this would be attempting to characterize the most common molecules to use their absorbance/fluorescence spectra calculate the concentrations of various classes of these molecules. With distillation it may not be be as useful since you should have already done the prep, but with primary extracts you could calculate the quantity of pigments and therefore amount of powders you need.
Edit: now that I think about it, I know you can do systems of equations to calculate the concentration of multiple overlapping absorbance spectra, can you do this for fluorescence? You could definitely get better info if you do it with two excitation sources to have a kind of binocular way to resolve some overlap, but for a single source I’m not sure.
Excited and honored to be presenting information on cannabis pigments at the American Chemical Society Western Regional meeting in October. This presentation will include some quantitative pigment testing, information on the immediate implications of consuming those pigments, and the usefulness of UV fluorescence for detecting pigments in a manufacturing setting.
I will make sure the presentation is made available to anyone interested here, though this group have already seen a lot of what I plan to present.
The Pigment Tracker v2 is also ready to go. If you have a v1 feel free to reach out to me any time for an upgrade!
viva Las Vegas!
Congrats on being accepted to present at ACS!! That’s awesome, and I’m happy you are going to be there speaking for our industry.
v2 Pigment Trackers are made of a more chemical and heat resistant plastic. Anyone who bought a v1 and ended up damaging it can reach out for a free upgrade.
Wookwerks is an awesome name
Have you or @AlexSiegel used any different excitation light sources such as Xenon? I ask because with Xenon, you will have a much stronger excitation and can measure the reflective spectra (literally the opposite looking spectra of an absorbance, most of the time). I wonder if you might be able to get more differentiated spectra’s between the cannabinoids.
I have played with them a little… Xenon or tungsten would be the go-to… Most likely xenon. Problem is they run really hot.
Am considering a multi source light that would generate a similar spectra but with low temp sources… Honestly though it would be pretty dope to use xenon directly.
We are working with an external light box with the low pressure sensor… The high pressure unit will be a bit more direct.
Yes they do run hot, usually why I’ve only seen them with reflective spectrometry. Instead of a constant excitation, you can “pulse it” (strobe light setting) that will help with overheating.
This is one of the best comment I’ve read all day.
I haven’t had experience with reflectance but it is super interesting to me! My brother is a geologist and likely uses it in his work. I’ll ask him what he thinks about it for potentially measuring cannabinoids.
My first thought is that likely the fluorescence of chlorophyll will be apparent under a strong white light and may effect the reading. Up to 550nm excitation chlorophyll seems to emit some 650nm light.
I’m thinking that could be a major determiner of spectra shape.
It would be interesting if you guys could create a quasi chlorophyll control standard that would help to correct that.
During my undergrad, I developed an sop as part of my Chem thesis of all things, for the biology department. I had a portable reflectance spectrometer used out in the field by avión biologists. Their overall interest was to study birds with iridescent feathers.
It would be awesome to use that reflectance spectrometer to measure the iridescence of cured bud. Could be a new solvent-less analytical method for determining trichrome quality, that is to say if trichromes on the bud are considered iridescent.
The Pigment Tracker flow cell is working very well. The low pressure c1d1 model will be available for purchase soon, likely in the new year.
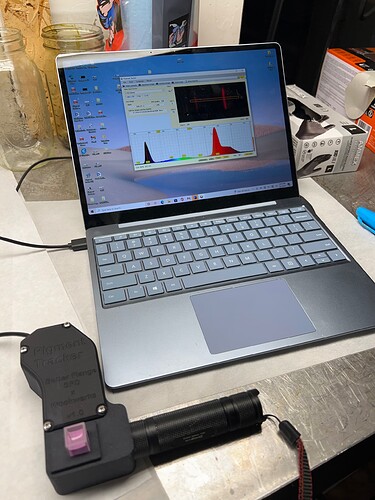
Displayed here is the transition from a very high concentration chlorophyll sample with significant turbidity (darkness) to a moderate concentration chlorophyll sample. As you can see in the video, when the chlorophyll is removed from solution the fluorescence of the chlorophyll as well as the other plant pigments become more visible.
More to come!