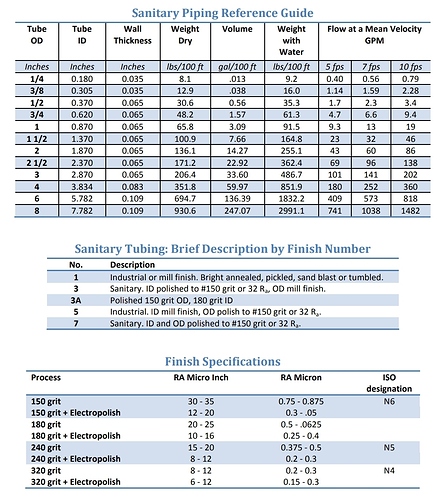
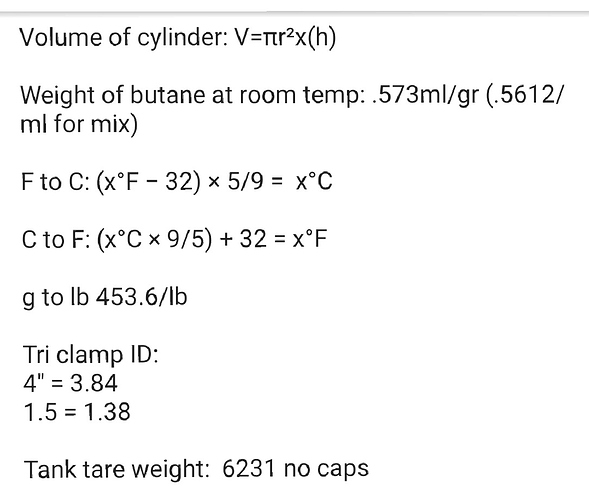
I have definitely considered this possibility (with every medium I ever tested), and I can say with 99+% certainty that this material is NOT observably soluble in butane, or probably any alkanes, at all. The 23" level in the sock never dropped (which kinda surprised me, since I figured it would at least settle tighter), and on the initial tests, I was pushing up to 6 runs through the same charge of media. That’s at least 600 lbs of cool solvent from extraction (probably around 450 fresh lbs, total) and well over 2400 lbs of submerged rinsing over at least 12-18 total hours of heated, high flow rate recirculation of 90 to 110°F butane at 50-60 psi! Oh, and this was done at least twice, because the first set needed fresh mol-sieve… so the solvent was about as waterlogged as could be, and neither dry nor wet butane showed any signs of dissolving the silica. All the sharp angles of the granules were still sharp, and field microscopic examination showed no pore size growth, poc-marks, nor any other signs of liquid erosion. There was also no sign of particulate matter or nucleation in any of the resin slabs (except a set about a month later when we got some new guys who were not trained on how to properly pack the cotton ball filter).
I suspect the same can be said for ethanol, even the 190 proof, wet and non-denatured potable stuff!
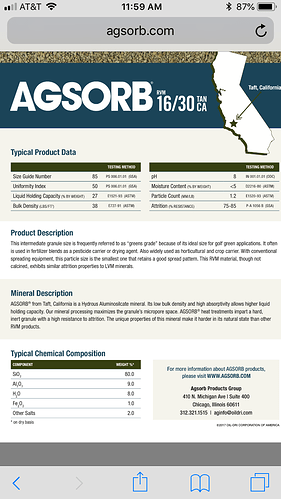
You may be asking why I did not use mass. Well, I have no idea how much mass of color bodies, pigments, phosphatides, lipids, wax or even desireable parts of the resin like bioflavonoids, water from the extraction, or even leftover butane, might have remained adsorbed to or absorbed within the “clay” after extraction, and even if I rinsed it with acetone, air-dried and cool vacuumed the solvent(s) out for safety, then vacuum baked for the same time and temperature as I did prior to using it, there would still be no definite elimination of any of those variables… not to mention weight loss in dust into the filters and all the container transfers!
Regrettably, it’s just not a very precise science when one is working with room-temperature-gaseous solvents, sticky plant matter and dirt, but I do try my best within (even slightly un-)reasonable doubt, especially where the safety of others is concerned!
Fortunately, that is a much easier and safer test to perform on ethanol… ah, damnit! I lent out my little vacuum oven! Grr!
Well, anyone can do the ethanol/silica solubility test with vacuum oven-dried silica and pure 190 proof potable ethanol, especially now that solubility in cannabis resin and alkanes has been ruled out! You can even microwave it a bit to heat it in the ethanol (only 10 seconds at a time!), reheat, cover and soak for days in excess volume, pre-weigh the vessel and a dry coffee filter and/or cotton ball for use in a funnel to catch the dust mass, etc!
And best yet, you can do a mirror test on the filtered ethanol, to determine if ANY solid residue is left behind! Just filter well and let it settle for an hour or so before sampling with a dropper from the top of the liquid!!
Hey, do y’all remember these?
Those are some test runs in reverse order;
#1 is bottom right run
#2 is bottom middle
#3 is bottom left
#4 top right
#5 top mid
#6 top left
And I knew it was done after run #6, because the starting color from this batch of old trim was not much darker than that, and we had recirculated solution #6 for at least 3 hours! We stopped recovery when the liquid level was about half where it started, so we could continue recirculation another couple hours.