Its more about safety and terpene retention than it is increase of crystallization.
Which alkane is preferred for washing the crystals ?
Pentane would probably be best.
Cold pentane
Thank you gunna give it a try
Where can I purchase pentane ? Can it be bought online ?
If you dont need a lot you can find it on ebay, amazon, esty… its usually around $75 a liter. Im sure people here can chime in where to get larger volumes cheaper!
I buy it from https://labsociety.com/lab-equipment/pentane/
People keep mentioning pressure as a variable to increase crystal growth rate or crystal size. Can anyone actually explain how pressures effect crystallization kinetics other than by preventing residual butane from evaporating?
You can recrystallize thca near instantly much like other “simpler crystals”
So with it being pressurized. It considerably effects the evaporation rate of the solvents in turn allowing for more stable crystal growth. With any crystallization the slower the evaporation the better growth youll have. Instead of precipitating everywhere around the same time causing more small crystals.
How would any solvent evaporate under increased static pressure? Do you slowly release the pressure to allow evaporation?
Can anyone explain how/if Henry’s Law applies with two or more different gasses, say with nitrogen and butane or bho solution, as the law states “the amount of a given gas dissolved in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid”
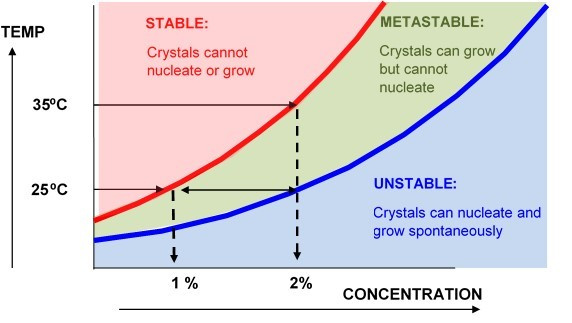
IMO, playing with and understanding saturation level is the most important aspect to growing crystals in a sauce. Understanding the metastable zone boundaries and how to manipulate your solution to those boundaries via temp and solute concentration. IMO, one of the the biggest hindrance compared to most crystal growers is the lack of a isolated solvent component to aid in that manipulation.
What we need for THCA and typical terpene/terpene mixtures.
Solubility phase diagram:
No it decreases under pressure. More pressure equals slower evaporation. Which in turn slow evaporation causes larger formations. The pressure is what we use to aid that manipulation. With out a closed vessel how do you manipulate your solute properly?
And if i find there is to much solvent left in for proper precipitation then i do do small burps.
Right we agree that under increased pressure there is less evaporation, the question is how less.
Is the partial pressure of the gas added to the vessel greater than the vapor pressure of the solvent. If it is I think that would mean there is no evaporation happening?
With out a closed vessel how do you manipulate your solute properly?
Without a closed vessel to manipulate our solutes saturation we adjust temps and concentration by either adding solvent or solute. Adding solute has the same effect as evaporating solvent, your concentration goes up. Adding solvent reduces concentration.
Not sure why a pressure vessel is needed to aid in slowly evaporating terpenes, unless one is utilizing butane as their primary crystallization solvent due to low terpene levels.
Trying this method for the first time. I’ve had my first batch of jars sitting in my freezer in a iso/dry ice solution with the loose lids. It says that I should be seeing a clear layer on the bottom but I am not seeing that at all. Instead I am left with a semi-dark non-transparent layer. What am I doing wrong here?
sounds like a lot of lipids and other fats. Did you dewax?
yea it can be, but it will also oil out if conditions arent right where as “simpler crystals” lik NaCl wont ever oil out so its not the same
I did not. I don’t have inline dewaxing unfortunately. Would it be bad to decant the butane solution into a seperate jar?
