How would any solvent evaporate under increased static pressure? Do you slowly release the pressure to allow evaporation?
Can anyone explain how/if Henry’s Law applies with two or more different gasses, say with nitrogen and butane or bho solution, as the law states “the amount of a given gas dissolved in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid”
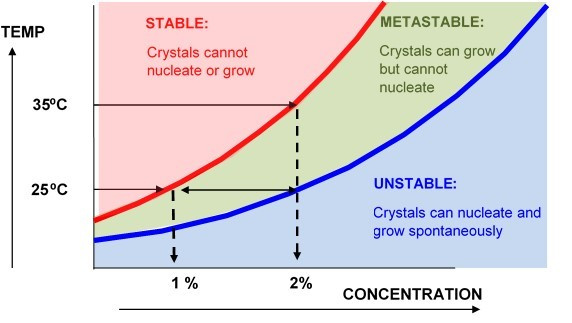
IMO, playing with and understanding saturation level is the most important aspect to growing crystals in a sauce. Understanding the metastable zone boundaries and how to manipulate your solution to those boundaries via temp and solute concentration. IMO, one of the the biggest hindrance compared to most crystal growers is the lack of a isolated solvent component to aid in that manipulation.
What we need for THCA and typical terpene/terpene mixtures.
Solubility phase diagram: