sorry about the spelling.
Just try it…220 F (certified temp measuring) sand bath
thin film in a test tube. It should come up to temp in a minute or two.
put about 50 mg in each tube…and do a time course.
run it for 2 hrs…taking a sample every 15 min…run TLC at end.
just follow production of THC and disappearance of THCA.
Look at my original statement…“about”.
I have NOT done the decarb proceedure, I describe, with a kg quantity of THCA.
But more times than I can count or remember with analytical samples. Nor, does it not mean I am not pulling samples from
kg runs on a wiped film or samples of Kg runs spinning at 90C for hours on a rotovap. My experience has been analysis of Kg runs…I put mg amounts in test tubes. Moreover, I do not always look for the 100% decarbed end point. OK.
Anyone with understanding of the physics and chemisty
of a 1 kg quantity of a viscous mass …would not in any way consider heating and maintaining the large quantity with a specified heat source …is anyway comparable to thin films in test tubes.
Your point is well taken. Everyone, doing scale production should
determine their own time course/end point.
Please describe the methodolgy you use for
heating and decarbing large masses of THCA.
This is not an issue of right or wrong, but one of pratical
significance at “scale”…please inform and we will all benefit.
There are salt issues about what you consider to be THCA.
And it all depends on how you prepare your THCA.
If you prepare the sodium salt of the acid moeity at neutral pH
would you expect it to “melt” and or decarb at the same temp as
the COOH form?
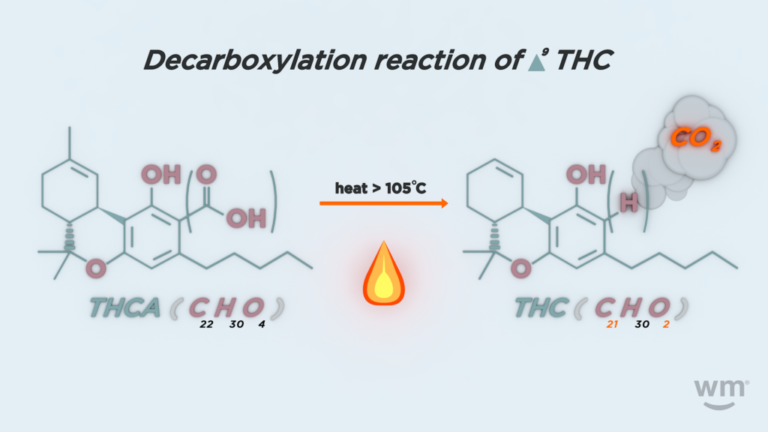
THC C21H30O2 314.2 157 314.6
THCA C22H30O4 358.47 105 220
CBD C21H30O2 314.22 160 - 180 320-356
CBDA C22H30O4 358.47 316 - 531 600.8-987.8
CBN C21H26O2 310.19 185 365
CBC C21H30O2 314.22 185 365
CBG C21H32O2 314.22 105 220
8-THC C21H30O2 314.16 175-178 347-352
THCV C19H26O2 286.41 220 428
Last number is B.P. in F degrees.
If THCA boils at 220F/105C do you have some magic method of superheating it? Other wise more heat only increases the rate of evaporation ( and or decarbing in this case) …it does not increase the temperature of the fluid to any great extent at atmospheric pressure. Capisce?
But then you have to consider…as the liquid turns to a higher mole fraction of THC…from decarbing, you begin to have a significant
mixture of a "THC solute in THCA boiling fluid…and the boiling point of THC being in excess of 300F…you would expect your temperature in the boiling flask to increase considerably (with excess heat source) near the end of run???
I assume you guys know what you are doing. You understand what I am saying?
Gobstopp did something interesting as I pointed out :
He decarbed in a high pressure sealed Parr reactor.
By confining the process at …he was able to get the side walls up to 220C and the interior pressure of decarbing liquid to 225 psi. It is possible the reaction could be over in minutes. In this regard, consider how the injection port temperature of a GC instantaneously decarbs THCA. This suggest a very possible way of fully decarbing in very short periods of time. A retort, high pressure steam. I am quite sure this method is applied at scale by some operations?
Twisted: "So both of you are fully decarbing crystalized THCa that has been mechanically separated under these parameters? "
did we say “fully decarbed”? No I said “about”…to contrast with the
very high temperatures Gobstopp was reporting.
I would assume any chemist would follow the time course…under his experimental variation…about. Especially if he is in charge of production at scale. Yes, about 220F and about 1 hr.
Depending on your experimental apparatus…it might take you an hour or more to bring a Kg of THCa up to temperature of 100 C.
Are we talking about decarb rates at the boiling point 105C or are we talking about heating a viscous mass from room temp to 105C?
Of course it takes you a long time.
I have asked…what is the meaning of “mechanically separated under these parameters”. I am trying to understand exactly
what you are putting in the flask to “decarb”?
And what temperatue do you “think” you are decarbing at?
yes THCA comes in D8/D9 forms just like THC.
not a typo
thanks.