I added 10% c-bleach to my RBF of a series of mixed tails. Did my best to push out all of the azulene, but my distillate still came out very dark. I had it tested by the testing company we go through and it came in at 69% delta 9 THC and 11% CBC. Am I missing something here?
Any input is much appreciated
no, your testing company is.
$50 says there is no CBC in your distillate & you made what is often referred to as delta 10 THC.
I’m not sure Steephill calling it such is definitive, but I’m certainly rolling with that moniker till someone with the correct tooling (yes, I’m looking at you @QGA ![]() ) tells me otherwise.
) tells me otherwise.
The classic where did my thc go post
One of the primary sources of issues is running a Vac/temp program that hits the material with too much heat too close to atmosphere. 10% is on the aggressive side as well. You can start with 5%.
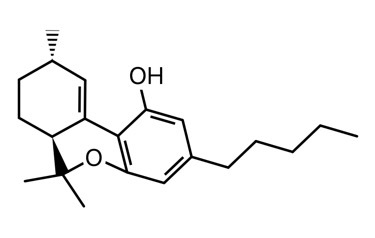
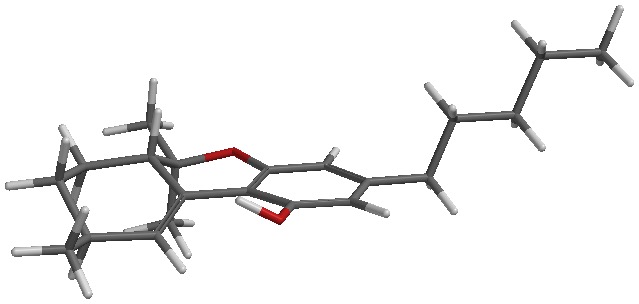
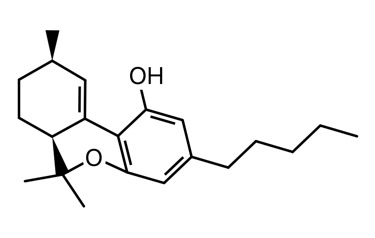
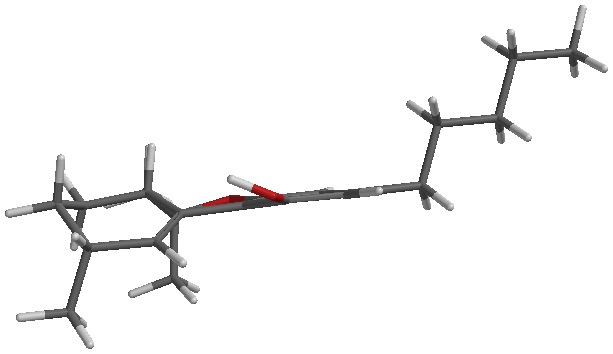
I’m tentatively calling it d10 or d10a/6a. There are two peaks that I see from degradation in my GC. This is also seen in LC. I’ve been told it’s not d10 by the scientific director of a testing lab that I trust. That only means to me that it might not be the same stereoisomer as the d10 standard they have. There are 4 possible stereoisomers, the diasteromers could have different boiling points and retention times. Or it could be a mix of d10 and d10a/6a (god only knows if each peak is a racemic mix of each compounds enantiomers).
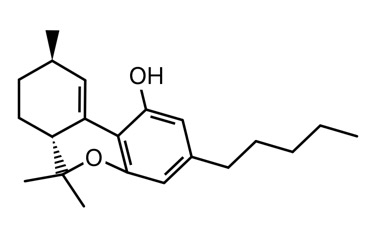
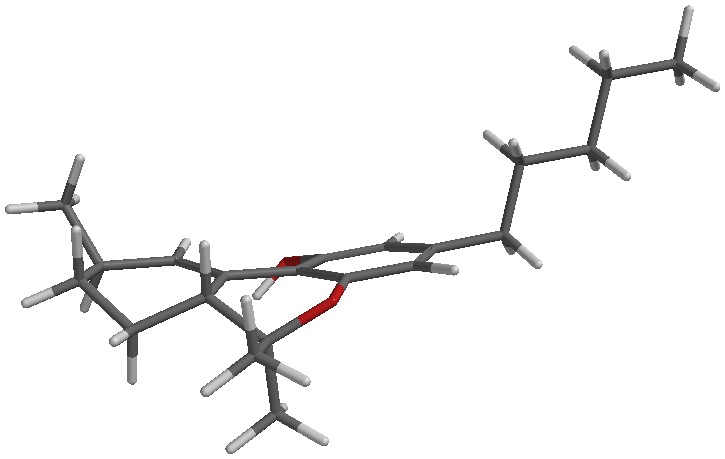
Clear as mud? I’ll attach a picture of each to my next post.
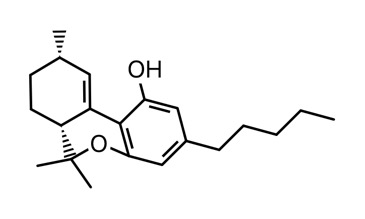
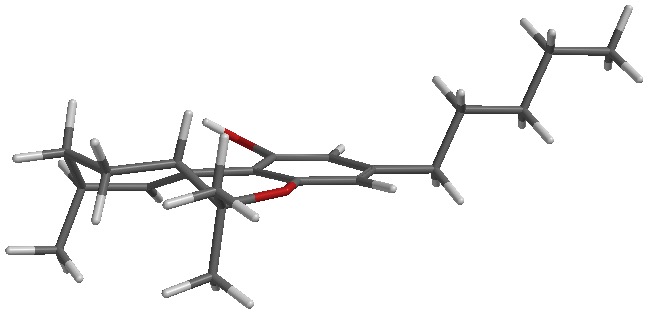
The sketch and the iSpartan minimum energy conformer of one isomer (S,R). I better have gotten that right…
Is there ANY confirmation whatsoever that these mystery peaks are d10? This community often mixes up hypothesis and fact, and I’m trying to understand if there is any actual data to confirm d10 exists in these samples or people are just guessing.
Someone should try and access (isolate or synthesize) all known cannabanoids and report: spectroscopical data, retention times, Rfs, etc. This would help clear up a lot of confusion.
Have you scoured the data dump?
I swear I stumbled across at least some of that data.
It wasn’t what I was looking for at the time, and I figured it was “easily looked up”.
Can’t find it again…
If it’s not there, maybe you should make that your PhD thesis? There seem to be a number of programs willing to wxplore that.
@Kingofthekush420 @MagisterChemist @drjackhughes
I wonder if anyone has stop flow UVscan or DAD capabilty on their
HPLC. I think tentative structural assignment maybe applied using de Backer’s reported UV scans from “Innovative development and validation of an HPLC/DAD method for the
qualitative and quantitative determination of major cannabinoids in cannabis
plant material,” UV lambda max analysis of these d10-ish targets, and retention time.
Is there a thread more appropriate to this topic ?
PS i cry when i see sri ![]()
Diasteromers of d10 might show up as discrete compounds on HPLC especially if the conditions are optimized for d10 analysis however that won’t tell you absolute structure. Need an NMR and/or X-ray crystal structure. The electronic spectrum is still useful data to have.
Why would the absorption spectrum change based on double bond location. UV spectra for D8 is nearly identical to D9. Your best bet for D10 is going to be NMR. IR could be useful since the type of olefin is changing but the fingerprint region will be garbage unless running a bunch or scans or having a high quality instrument.
Be nice to verify m/e as well. I think bathochromic hypothises would be tentative with phase affinity untill further verification can be made. Polarimetry and IR data too…
You are effectively extending the pi system in d10 and d6a. The bond is conjugated with the ring. The double bond from d8 to d9 remains unconjugated.
potassium tert-butoxide
Love the sharing. What solvent do you prefer. Are you buying or making fresh?
hexane, and Reagent grade
I just ran calculations on the two (D9/D10) and there is small red shift. Most people wouldn’t be trained to see this small change.
Worked on predictiing subtle and drastic changes in electronic spectra in grad school. Trying to tune macrocycles, most of this is unpublished.