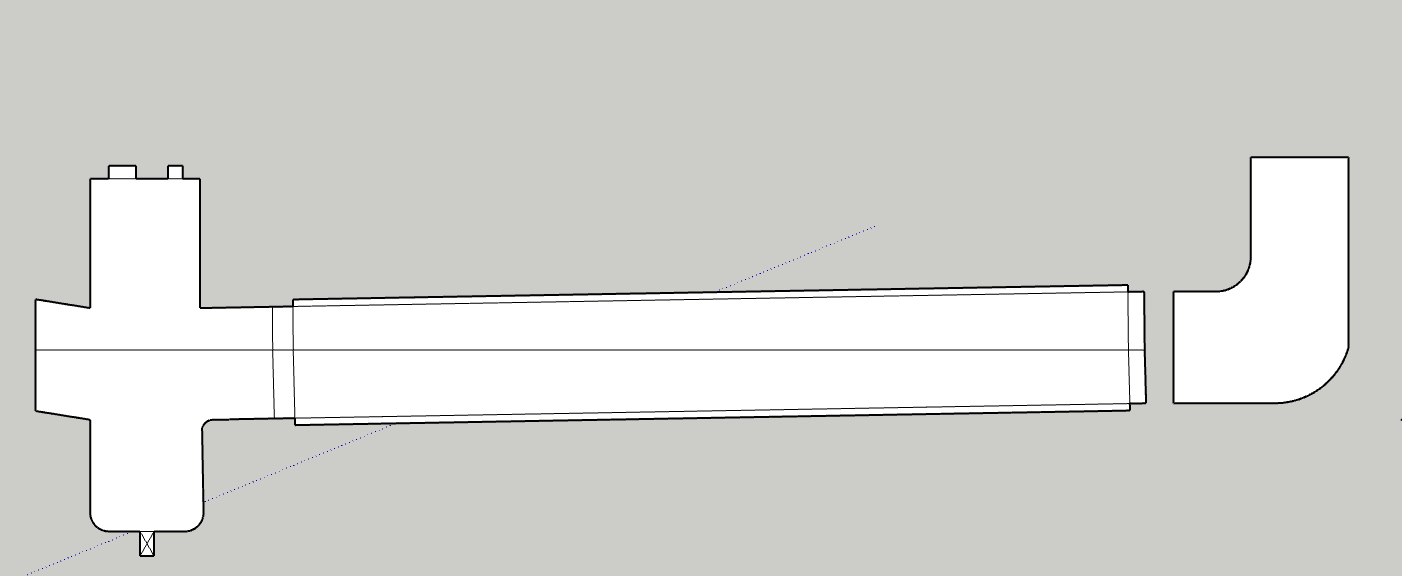
The bubbles are the main concern for sure, I would be shooting for a half fill at most if i was to flood the entire collection, which at this point isnt the plan.
I would have a 6 or 8" cross on one end of the horizontal collection tube, that would serve as lower end collection area, either platter or pour out or redirect to honey pot. The side of the cross would be for a sight glass, ideally a modified cross with a opposite downward tangent slope so no hold up occurs due to the slope. The top of the cross would be for gas recovery path, injection, prv etc, “the lid”. The other end of the collection tube, the high sloped side, could be blanked off, sight glassed, or elbowed upward with an injection port to slope and further thin the incoming solution.
The volume available for foaming or bubble expansion could also be increased with a vert spool on the top of the cross.
pic for reference:
For messy clean up, if necessary my go to is the reflux method that I first came across from one of @SamuraiSam’s many, many helpful posts. Ive used it a few times, it works well.
Ive seen them but didnt know their function. If I understand right, its removing the “specific heat” requirements so we have to deal mainly or only with, “heat of vaporization”.
Which can open the dialog and question to best practices, and why the convo turns to evap solutions like @CuriousChemist22 has laid out about falling films and efficient usage of surface area, etc.
There is a specific thread on the topic,CRC active butane falling film open source. sure wish @Waxplug1 would drop a line or two about their spray lid…!
Also, be sure to check out @Graywolf’s concept configuration for a horizontal / increased surface area collection, on his website post 15.6 ASME Pressure pots - GrayWolf's Lair at the end of the article.