Ok wow, this has been quite the thread to read. I have a handful of hypotheses as well as background information to add to hopefully illuminate some new pathways.
The first ~3600 posts more or less contain the ‘it’s a blue impurity’ hypothesis (azulene, ph active xanthophylls etc.) and I think it’s been said enough. I do want to acknowledge @Kingofthekush420 's hypothesis of blue terpenes by UV degradation (post 1606) as I think it has the most merit in this school of thought, even though I don’t think it’s the most accurate. The main reason I believe it to be false is that when the crystal is melted, it is no longer blue, implying this is structure dependent.
There are several known phenomena that can cause substances that do not have an appreciable visible spectrum to emit visible light; in this case crystalline THCa being blue. There’s two ways we can get here. Generally speaking, we can either scatter the frequency of light we are trying to see (blue), or we can absorb the complementary color of what we want to see (absorbing orange). Let’s discuss the possibilities.
Scattering & Fluorescence
There are a few things that could be happening here in the scattering hypotheses. One could be a quantum dot type of phenomenon where there are small terpene crystals that are embedded in the THCa lattice that are fluorescing in the visible spectrum (appendix 1); basically, the band gap of the particles is the same wavelength as the color of the blue we see. A good way to test would be just shining a UV light on it.
This is unlikely, but a similar principle is that there are some crystal subunits that are on the order of 450-495nm throughout the lattice that are scattering blue light because they are similar in size and therefore interacting and scattering with it. This could be induced with specific crystallization parameters, or like oswalt ripening, which was mentioned several times very early in the thread by @Kingofthekush420 (post48) and @TheWillBilly (post 25) himself! (Though willbilly saying this makes me think that it’s not the case since he is trying to monetize this tek ![]() ) In this case, the amount of these particles would have to be low because the particles would still interact with visible light and make the crystal cloudy, so I don’t think this is less likely, but not improbable as long as tight control can be maintained over particle size, but those are 2 cases where structure alone gives rise to color.
) In this case, the amount of these particles would have to be low because the particles would still interact with visible light and make the crystal cloudy, so I don’t think this is less likely, but not improbable as long as tight control can be maintained over particle size, but those are 2 cases where structure alone gives rise to color.
Complementary Color Absorption
One of the ways our brains process color is complementary color elimination where the absorption of one color will make the substance look like the complementary color (ex. absorbing orange light will make something blue). Since THCa and the terpene(s) in question are not pigmented, we have to take a more advanced approach to the physics here. This is also my best guess and goes hand-in-hand with @TurahTurah 's co-crystal hypothesis.
Charge Transfer
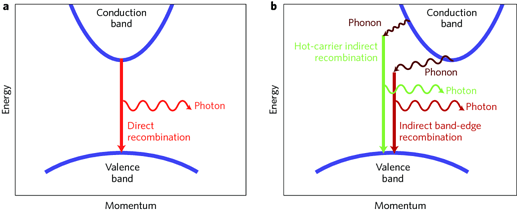
To understand this, we need to look at the properties of semiconductors and insulators in general, specifically something called the band gap. The band gap is a term used for semiconductors and it describes the energy required to excite an electron from the ground state (the valence band) to an excited state where the material will conduct current (the conductive band), or more generally, the excited state. However, semiconductors are merely insulators with a small band gap, so this term can be applied to any insulator (organic compounds). Though many semiconductors are inorganic, you can also have organic semiconductors, and this is where you get organic led (OLEDs) and organic solar cells.
Figure 1: A simple illustration of the band gap concept.
The main issue here is that our energy gap for THCa and terpene(s) are in the UV spectrum, so we can’t get any blue color since our band gap is too wide; the energy required to excite the THCa molecule is too large and absorbs in the violet-UV range (giving a translucent to pale-yellow color). What we need to do is change the band structure (orbital structure) of the pi system to lower the energy enough to absorb orange light.
This is observed in what is called a charge transfer co-crystal. When a photon comes in and excites a molecule, it generates what’s called an exciton (an electron-hole pair), and the size of this exciton is roughly the same distance as molecules are spaced apart, so if you have one material for transporting holes (lack of electron) and another that can transport electrons, you effectively lower the band gap of the molecule, thus changing the color!
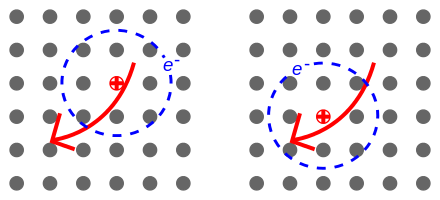
Figure 2: A sketch of an exciton, one dot represents one molecule the arrow shows movement of the exciton.
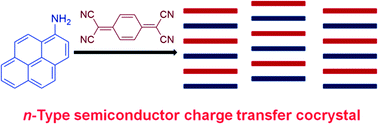
Figure 3: A crystal lattice of 1:1 co-crystal of 1-aminopyrene and TCNQ and likely absorbs most of the visible spectrum.
I have personally worked on a very similar system of figure 3 (pyrene/TCNQ), and they are transparent and green (respectively), but when you mix a solution of the two together, the solution turns black as the CT-crystal forms and the band gap changes.
Applying this to THCa Diamonds
Now with THCa, we obviously aren’t 1:1 THCa:terpene, its 99.9% THCa, in this case there is a small amount of terpene that is still forming co-crystal, but in this case the co-crystal itself is doped into the larger crystal structure, which makes sense since pure co-crystal would absorb very strongly.
Now in order to absorb orange light, we need to shift the band gap from the UV range to orange light (quite a large energy difference). THCa has a rather electron donating aromatic ring, so a good first guess would be to pick an aromatic terpene that is very electron withdrawing. If I pull out my handy-dandy Eybna Terpiodic table, my best guess would be cuminaldehyde, though some flavonoids like isovitexin could be other candidates.
That may be a bad guess though. Crystallization itself is more art than science, especially when you start going into co-crystals and it could be non-aromatic like piperatone, pulegone, or carvone or less electron donating than expected like menthofuran, m-cymene, or cupramene. It could also be not aromatic at all!
A great terpene chart by Eybna
One final note is that other terpene combinations can lead to different band gap changes, and therefore different colors! So, you very well could make different colored diamonds that are truly cannabis derived!
Edits: Hella typos and adding additional info to be more concise and accurate.
Appendix:
(1) Quantum dots are basically just particles of very uniform size that range on the order of (but not limited to) nanometers; they have other interesting properties, the main of which being semiconductor behavior where the actual size of the particle determines the band gap (energy between ground and excited state), not the electronic properties of your chemicals. Most notably, quantum dots will fluoresce when exposed to UV light. Here is a great thought emporium video where he makes quantum dots out of table sugar!
Colorful Quantum dots Made Using Table Sugar - YouTube