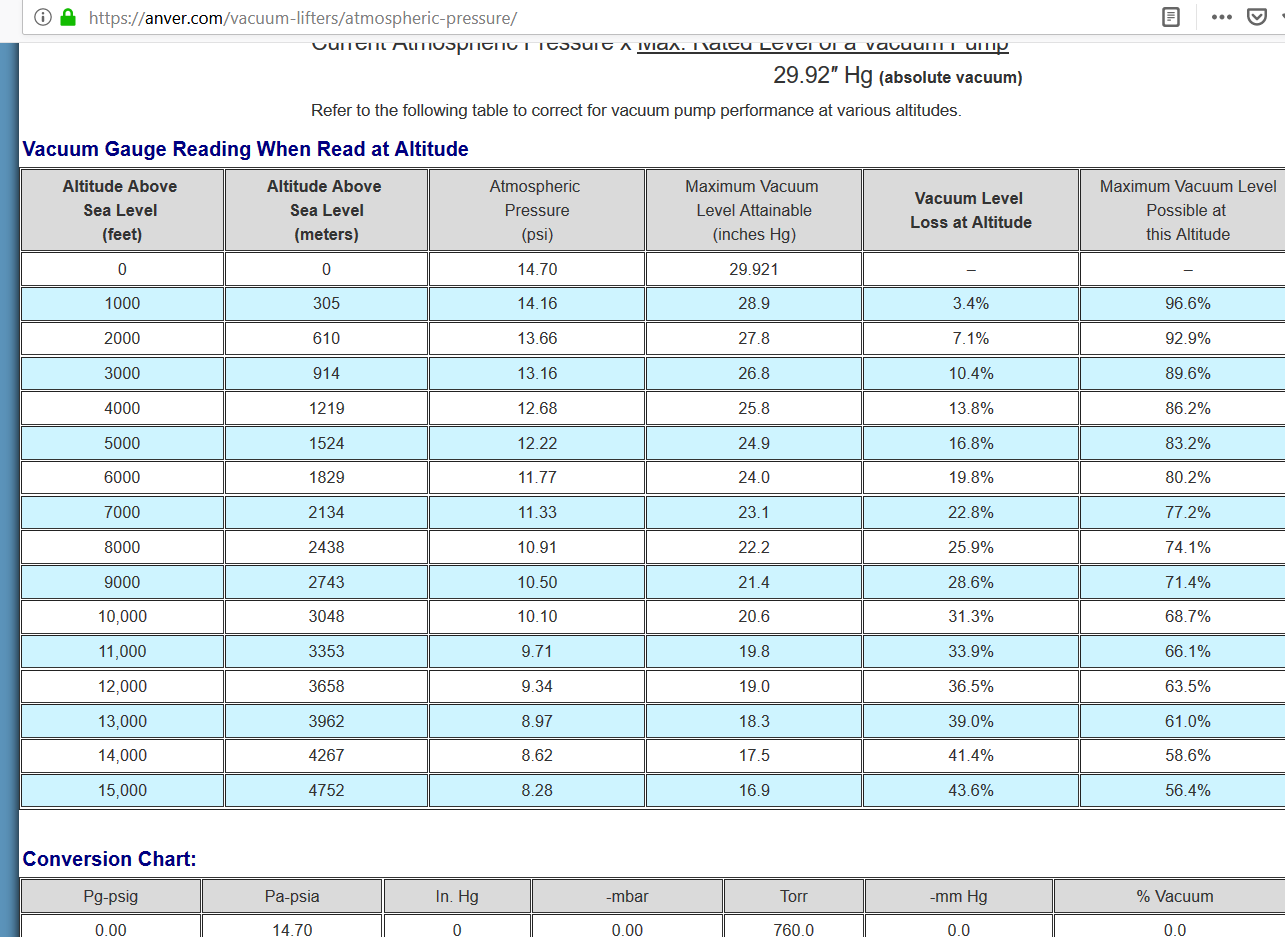
the higher the altitude the less pressure it takes to cause evaporation. At sea level you have alot more pressure holding the solution together then at high altitudes. Higher altitudes have thinner air and lighter air which makes evaporation easier. I use to live at sea level, now I live over 4000 feet above sea level. Ive noticed i cant pull my roto down as much because the air is thinner up here which means the vapor moves through my roto easier because its not so dense. The less dense air makes my vacuum pump less effective too because the air particles here are bigger so your vacuum doesnt move as many particles when you have light air compared to dense air. Heres a chart I found on the internet that shows total loss of vac at certain sea levels. Look at 4000 feet, i have a 13% loss in vac because of the less dense air. That means I can only pull down to a max vac of 25.8 inHG. I am by no means saying this chart is accurate, its just an example of how vacuum is effected by altitude.