What temp did you say it dissolves to liquid at?
No. Nor suggesting it is not.
plant origin is certainly a decent bet. @Photon_noir suggested that also.
It failed the “is it a cannabinoid I recognize” test on the GC. Not dissolving in alcohol until heated got me most of the way there…
Beyond that I know nothing. (Not even if it’s water soluble)**.
It also it seems did not we keep enough around to explore much beyond this. I’ve got two 70mg samples in dirty solvent.
Don’t know if what @Dred_pirate has is the same critter. Certainly know one way to find out…
Edit: Certainly a biological source for “it burns at the FID” & “is a white solid at rm temp” makes more sense than “it was in the solvent!”…
** Actually I’m reasonably sure that alconox and water failed. I initially assumed I was dealing with CRC media.
I can definitely get some in your hands to find out
What temperature was the alconox and water?
Is there any way the unknown peak could be eluting so close to the solvent peak that you’re missing it entirely?
No absolutely not.
I’ll get a chromatogram up tomorrow.
Dissolved in ethyl acetate (thanks @Beaker!)
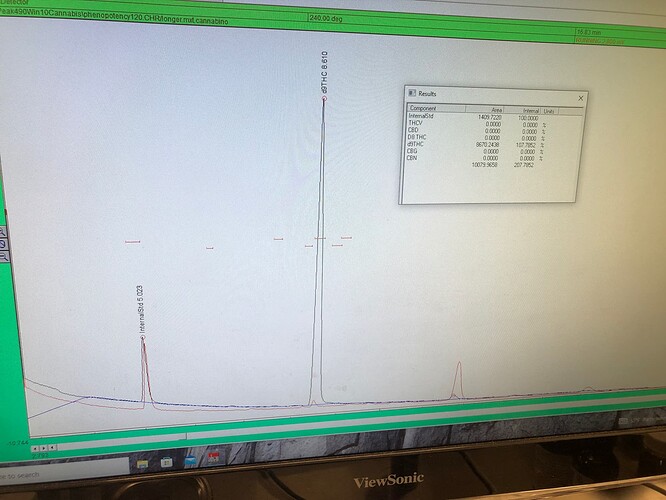
Looks like it’s THCA!!!
What do you mean it’s THCA. Wouldn’t that have shown up on GC?
Sorry. Let me rephrase:
Once in solution, (ethyl acetate), it flys on the GC and looks to be THCA.
Which one is NOT what it looked like injected in ethanol.
Looks like thca on hplc too.
Huh well that’s odd. Seems to crash much easier than I’m used to.
Indeed. 70mg THCA crashing in 40ml of alcohol at rm temp is NOT normal.
3rd party tested THCA vs “WTF”.
Both in same solvent (home despot denatured ethanol).
72mg THCA vs 70mg “WTF”
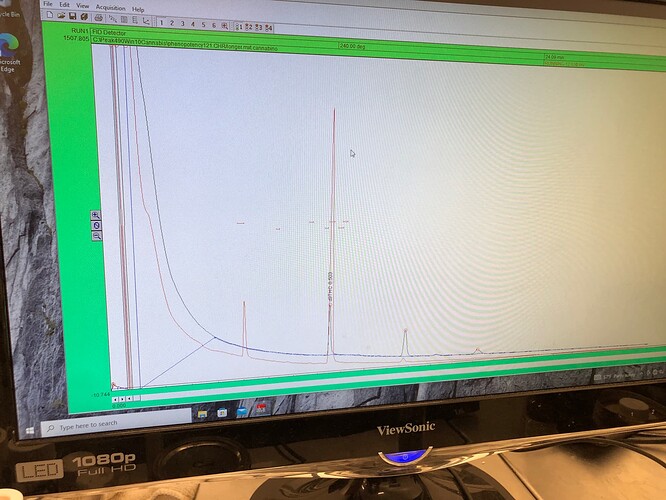
samples from above run on GC…
Red was pulled from “ppt free” region of vial, and would be expected to be “depleted” of whatever it was that precipitated.
certainly not much where one would expect THC(a) to run.
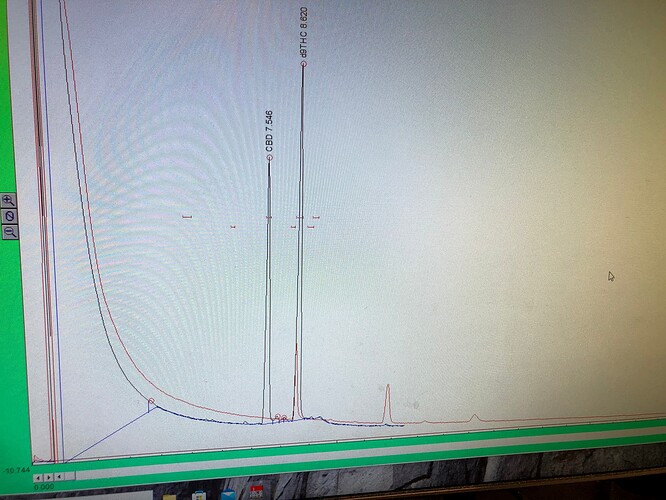
This is thca + infernal standard (methyl stearate) in red, and a sample we prepped for/ ran on the HPLC in black.
that sample looks like (95+%) thca via hplc…
Sample was bought up in ethyl acetate, then diluted 20:1 in 200 proof ethanol for hplc.
Red is the hplc sample shown previously (diluted in etoh)
Black is ~20mg each “wtf” and CBD isolate.
(20mg +/- 4mg… I need a better balance)
“WTF” certainly seems to co-elute with THC(a) here…and seems to give a similar response (w/w) at the FID (so most of that 70mg is probably carbon).
that’s assault!
Why yes, it probably is…
might even claim it was a salt with a weedly depend.. ![]()
Currently evaporating the ethanol off one of my initial samples to rule out that I mixed things up somewhere.
Hmmm, could it be used to selectively crash thca from cbda rich hemp crude?
What’s the origin of the gas ya’ll use? Would it be safe to assume that more and more gas, especially now, comes from fracking?
Hmm. Seems like you must have something in there creating a co-crystal. Quantitatively speaking can your HLPC tell if this solid is 100% THCA by dry weight or is there something else in there?
Balance is not up for that…had less than 10mg to work with for that sample….and now I’m out.
Dang. So how exactly did you isolate this in the first place? How can you get more?
Magi: what would ever make you think “co-crystal”?
“So how exactly did you isolate this in the first place?”
It emerged from nonPolar butane , …i.e., butane works…
Let’s not over think things here:
The wide variety of effects associated with a competition between intra- and intermolecular H-bonding can be illustrated with salicylic acid. In the simplest case of salicylic acid crystals, the carboxyl groups of the molecules form dimers while their hydroxyl groups form intramolecular H-bonds **This structure remains qualitatively valid in an aprotic solution when the dimer is deprotonated. **
(Now that is interesting)
In contrast, when the number of competing interactions increases, the co-crystals of salicylic acid exhibit polymorphism and different solubility. These changes are critically important for pharmaceutical applications. Besides that, the intramolecular H-bond in salicylic acid derivatives can be controlled through intramolecular steric effects. In the crystalline salicylic acid, the O…O distances of this H-bond are about 2.62 Å Is this a general trend that can be expected for other molecules’ structures?
Did you say. “Co-crystal”…?
Seriously, for all Butane experts on 4200, WTF have you been thinking all these years extracting with Butane from trichomes containing in excess of 4000 organics…nice little R-COOH molecules floating around in liquid butane? Ready to crystallize by accident?
Magi…you’re good to go…keep thinking…please.
Cyclo…is just trying to confuse everyone with data.
We don’t extract with butane to get thca. There are better ways if all your looking for is thca. But if you want the fire terps, using other solvents doesn’t work really well.
I have yet to read through this thread, but we have been seeing a lot of fast crashing since I switched to GasLogix…
Not sure if it’s related at all or not.
@lefties.cannabis I’d recommend the It’s Not Isobutane?! thread as well
There’s been myriad theories here, but I like the amine/NH3 theory the most
Their gas loves to fast crash. It was the gas i was using when my issue started, I still havent received any COA’s from them after asking for months so I’ve since switched to another supplier.