Not a chemist, need an assist figuring this one out…
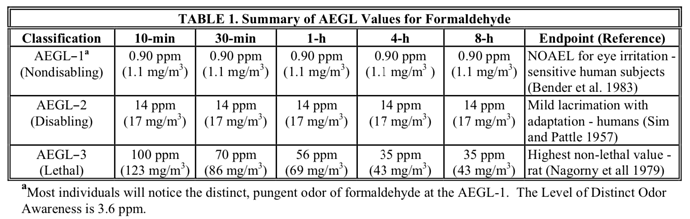
Our decarb reactor appears to have become homicidal. Near as I can tell it’s emitting formaldehyde!
Duratherm swears that’s just not possible. I’ve got no other explanation. They’re happy to test our fluid, but their testing consists of “flash point, viscosity, and total acids”, which doesn’t seem like it’s likely to solve this.
I could use some help setting up a testable hypothesis or three to figure this out.
Here’s what I have so far:
I asked the all Gnowing One about the production of formaldehyde because I was pretty sure I caught a whiff of it. when ASKED to associate the thermal degradation of siloxanes to the production of formaldehyde I got the following


Pretty clear that aldehydes are a KNOWN degradation pathway for poly-siloxanes….just no obvious explanations for why it might happen at low temperature….(until I ASKED)
When I drop the requirement for formaldehyde, and ask about low temp decomposition, I get
Which, although it doesn’t mention Form-aldehyde explicitly, definitely lists it as a possibility.
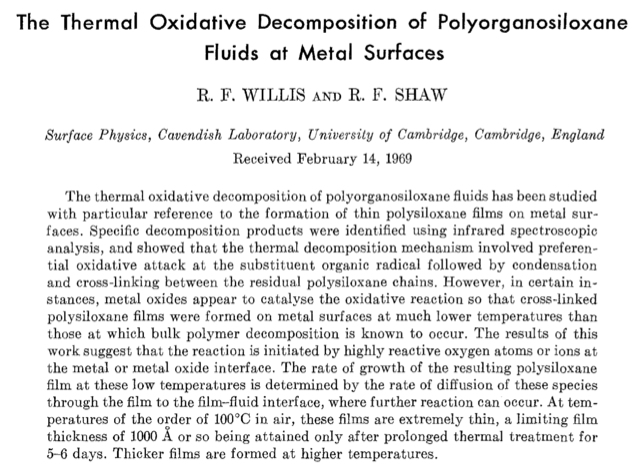
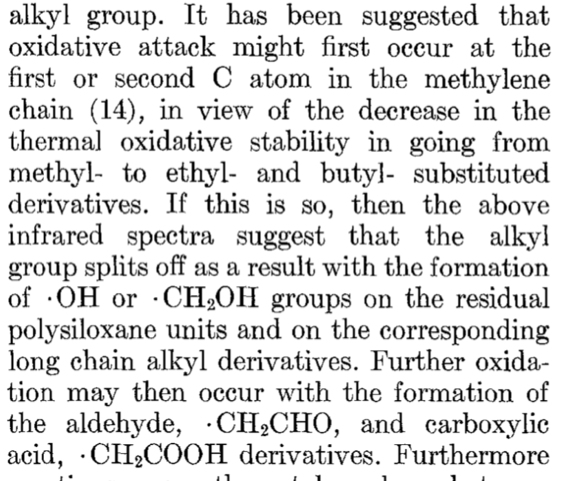
Assuming you’re behind a paywall on that one, you can obtain the full text via
Here is the rig in question
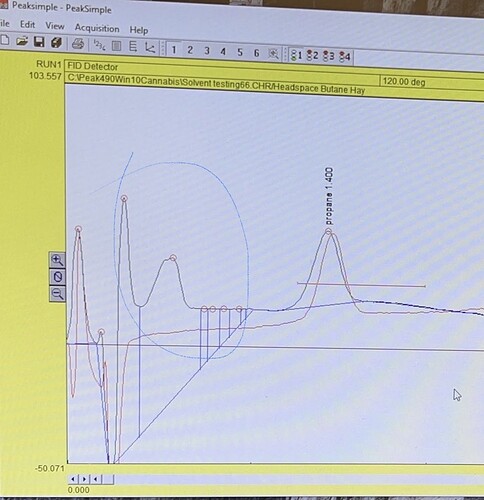
And here is my first attempt at analysis. Head space sample taken from the circulator.
Two unidentified peaks between water and propane. Which is where I imagine H2C=O would likely elute.
I’ve got a brand new 5gal pail of duratherm, and was planning on new vs used +/- some welding slag to see what I can find.
My best guess is that the insides of those jacketed vessels resembles this nonsense, and that chromium oxide or nickel oxide make great catalysts (they do!).

Edit: formaldehyde “standard” incoming.