Hello everyone ![]()
Is it possible to seperate Ethanol and n-Heptane with mole sieves?
As far is i know an what i have found in the forum, it isn´t possible to seperate Ethanol and n-Heptane trough destillation. The azetrope of these 2 components has a lower boiling point as ethanol.
I want to experiment with a few things. I want to know if it is possible to swap the solvents after extracting.
(Because of the law, 0.3% THC can`t be exeeded.) If i put 10 litres of n-Heptane to 10l of Ethanol, how do i get the Ethanol out?
I tought of someting like that. This can be used for dehydration. Havent tried it yet. Anyone of you?
https://www.m-chemical.co.jp/en/products/departments/mcc/ifm/product/1201039_9112.html
Water can be “caught” (pass trough) with 3A zeolith. Ethanol can´t.
Can the same done with Ethanol and Heptane? n-Paraffins have 4.2 Armstrong if I´m right.
Ethanol is below 4 Armstrong. ==> As a result, it should be possible to seperate them with 4A Zeolith !?
Thanks for the answers.
yes, it is possible to swap solvents.
evaporate one entirely, then add the other.
can you explain how does separating those two solvents relate to the 0.3% THC requirement? (where are you performing this work?).
Angstrom… that other guy walked on the moon.
Wasnt there a trumpet player…Armstrong (Louis) as well.
When I can swap the solvents without evaporating, the THC is under 0.3% at any time.
The filtering & winterisation is done before the heptane comes in.
When the crude is dissolved in heptane, i can take out the THC with mole sieves or with an HPLC (==> i dont need a reverse phase HPLC) We are considering to build an HPLC ourselves since it is not that difficult.
I am from Austria.
![]()
![]() … stupid misstake. I hope you can forgive me
… stupid misstake. I hope you can forgive me ![]()
There was even a cyclist.
Mol sieves dont remove thc. It removes/absorbs water.
Edit
Iirc chromatography does. Or a conversion.
does Austrian law require that you never exceed 0.3% THC?
US law is being interpreted as “0.3% THC in final product”…with the understanding that it may be considerably higher during production.
Not sure if anyone has actually tested that interpretation in the courts yet.
http://www.ss-pub.org/wp-content/uploads/2018/09/BCR2018043001.pdf are using 4A sieves
The main objective of this study is the recovery and recovery of zeolite 4A already used for drying natural gas
suggesting your strategy is probably viable.
The legal text states that it isn´t allowed to exeed the THC content at any time… Shitty hemp laws !!! ![]()
Has any one tried molecular sieve cartridge like mentioned above for dehydration of ethanol?
Does anyone have more information about the Angstrom of n-Heptane? I asked google but didn’t find that much.
Louching the cannabinoids out of ethanol and into the Heptane is a way you could probably avoid having concentrated THC ever present in your lab. Also it’ll save you a distillation step at the cost of another one (reproofing)
look on the bright side. At least the law doesn’t state you’re only allowed to extract from the stems/roots/seeds (Japan I believe).
my only routine “solvent swap” is from Ethanol into MCT, which is trivially accomplished with heat.
@SidViscous &$@^(!)$!(*@ loucher! ![]()
![]()
![]()
How do you mean ?
WTF … Japan is hard
MCT Oil !?!? - I “need” concentrates too.
Full spectrum without THC. I will find my way.
add water to the ethanol.
cannabinoids will fall out.
heptane doesn’t pick up (much) water. will pick cannabinoids up.
cannabinoids in MCT sell well and are easy to achieve. low hanging fruit…
how is it full spectrum if you remove the THC?!?
If i do that (Water to Ethanol), the THC content in the curde is still above 0.3%THC
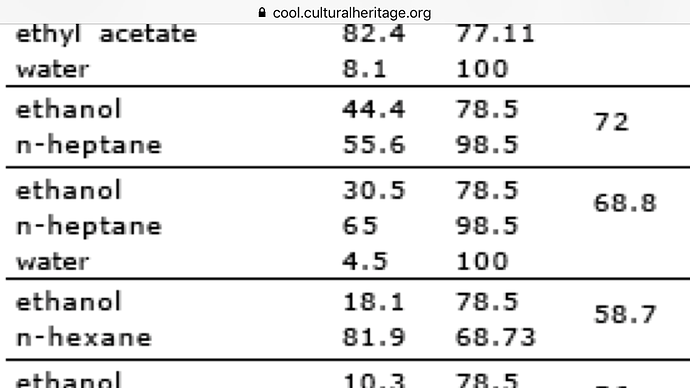
Which azetrope is stronger ? Ethanol-Water or Etanol Heptane? If i put enough water in the Ethanol and then the Heptane. Will Heptan Ethanol still make an azetrope? (Fast question… havent looked deeper into myselfe for now)
It´s our “Austrian” Full Spectrum …
I’ve not louched into Heptane, and I (now) recall that ethanol/heptane/water DO form a ternary azeotrope. see @photon_noir’s Ethanol Azeotropes - Attn: EtOH cannabis extractors!
I have watched @tweedledew’s process (into pentane). THC content would only exceed 0.3% if you actually centrifuged the ppt out. which is not the object of the game.
Use methanol
Add so much heptane that when azeotrope is boiled of what remains is a
Heptane/ cannabinoid solution
At 0.3% thc
For extracting or instead of heptane ?
I want to avoid methanol … its carcinogen.
How much Heptane is needed that only heptane will remain ?
Do you think it is possible with mole sieves ?
Otherwise i need a biiiig distillery.
And heptane isn´t that cheap.