A never never land of nano-micro scale…spontaneous aggregations somewhere between micro-emulsions and micro- micelles…“the louche” of pure cannabinoid, ethanol and water
lives in an emergent world of it own.
How to explain microemulsions formed by solvent mixtures without conventional surfactants https://doi.org/10.1073/pnas.1515708113
This PNAS article summarizes some “fantastic work” by a group out of Germany; the “theory” as to what exactly explains a “louche” is only about 5 years old!
““The stability of “detergentless” micelles or microemulsions in such mixtures was proposed in the pioneering works of Barden and coworkers [Smith GD, Donelan CE, Barden RE (1977) J Colloid Interface Sci 60(3):488–496 and Keiser BA, Varie D, Barden RE, Holt SL (1979) J Phys Chem 83(10):1276–1281] in the 1970s and then, neglected, because no general explanation for the observations was available. Recent direct microstructural evidence by light, X-ray, and neutron scattering using contrast variation reopened the debate. We propose here a general principle for solubilization without conventional surfactants: the balance between hydration force and entropy. This balance explains the stability of microemulsions in homogeneous ternary mixtures based on cosolvents.””
Deep dive for those interested:
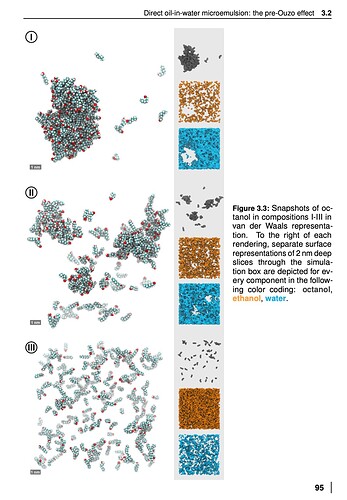
A thesis by https://epub.uni-regensburg.de/37989/1/2.0_comp.pdf
(Sebastian Schöttl)
“and then, neglected, because no general explanation for the observations was available.”
sound familiar???
14 Likes
It was due to helpful discussions with @anon64373531 that got me to
research deeper into this subject…he deserves credit and thanks.
the “louche” stands on it own.
8 Likes
That thesis is really interesting, gonna take me a while to get through all of that. Most people probably want to start at chapter 3 for the surfactant free micro emulsion section.
These sliced figures are cool:
6 Likes
they are spontaneous aggregates that form in ternary solvent systems,
just substitute your favorite cannabinoid for the benzylic acid (BA) in their models.
they really are not micelles, just micelle-like…but also colloid-like …
yes…combined with the concept of hydrotrope.
I have been working with the cannabinoids and ethanol water systems off and on for 7 years. But I had been thinking micelle. When working with purified cannabinoid like
crystalline CBD , water and ethanol…you can be sure you are working with a “surfactant free micro-emulsion”., however when you are working with a primary ethanol/water extract of
trichomes for instance, you can be sure there are additives such as palmytamide which
adds complexity to the structures acting as hydrotopes or surfactants.
This new work broadens the thought horizons of “solutions and miscible fluids”; and falls
squarely into practice and art of Cannabinoid manipulation.
Perhaps, get the concept of hydrotrope (types A and B) pages 8&9 of thesis clear first.
10 Likes
This all sounds really cool and probably really important…
Wanna dumb it down a bit? So I can… Tell a friend… What this means for us?
6 Likes
This is sort of like trickle down practicality.
It took all the chemists in History to come up with the theory in 2016 or so to explain the phenomena of the “louche.”
So the subject is not intuitively “obvious” even to Ph.D. trained chemists. Unfortunately, the details may escape many or exclude many, less-trained from the discussion.
So for a trickle down experience: @tweedledew, just take a gram of purified thc, put it in 10ml of alcohol. Take 1 ml of that solution (1/4 tsp) and pour it into a juice glass of water…and down it. Wait half hour or so…and report back 4200 on the “dumbed down” version of this subject. That is the low tech, instantaneous version of water soluble THC.
Yes, and “Butane works no theory”
3 Likes
Gotcha, I do a modified Louche but with an alkane to make sure the goodies find somewhere to go. So I suppose it’s perhaps not technically a louche if the cannabinoids don’t stay in the water/alcohol solution
2 Likes
OK…yes I worked with that system for a number of years…
I am assuming you are starting with an alcohol extract…adding water…
then back extracting with Hexane…(and a few extra tricks we don’t need to mention)?
3 Likes
If I’m extracting live I’ll use an alkane, then LLE to methanol, then concentrate, then likely add pentane, discard water mix and evap to preserve Terps.
Im extracting dried then I use methanol, run a small hep/hex layer on top to snag fats, then water and pentane again, unless I’m getting real fancy on the crystallization end.
6 Likes
Interesting approach. they allow methanol in your State?
because methanol work well in regards to how you are using it.
and I am sure you understand the ethanol issues in those applications
I have done a bit of amorphous crystallization of C-acids from MeOH as well.
3 Likes
Tricky part is when you can’t use methanol and you’re forced to use ethanol by regulations.
so you have hands on experience working with the phenomena noted above. I’ve argued for a few years it was a micelle…
But it is something “new” in terms of descriptive non soluble suspensions…
Regards,
1 Like
Yeah, I don’t have any regulatory bodies involved 
I find when doing water washes on my heptane layer I’ll get quite the emulsion, especially when it’s highly concentrated.
Gotta use isopropyl to break that shit up, although likely other alcohols would work too.
1 Like
Anybody crystallizing using a louche method?
4 Likes